Method for preparing optical pure p-hydroxy phenyl glycine by separation method
A p-hydroxyphenylglycine and optical technology, which is applied in the field of preparing optically pure p-hydroxyphenylglycine by a splitting method, can solve the problems of increasing the requirement of a splitting agent, increasing the cost of the splitting agent, etc., and achieves the effect of reducing the production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Add 167g (1mol) of racemized p-hydroxyphenylglycine to 450mL of ethanol / water solution (volume ratio: 1:1), heat reflux to 276.46g (1mol) combined cyclic phosphoric acid resolving agent until the solution is clear, and stir for 1h. After cooling to 50°C, crystals were precipitated, filtered, washed the filter cake with 56 mL of ice water, and dried to obtain 218.4 g of filter cake with a melting point of 230°C-232°C.
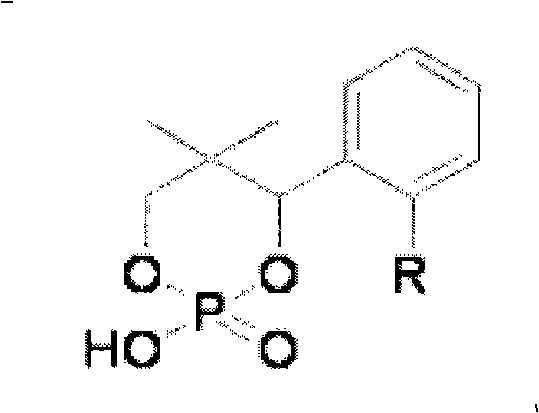
[0031] The main resolving agent in the combined cyclic phosphoric acid resolving agent is (-)-CP2, the auxiliary resolving agent is (±)-CP3, and the molar ratio of the two is 99:1.
[0032] (2) Dissolve the above filter cake in 50 mL of 36% hydrochloric acid, stir at room temperature for 4 hours, precipitate a large amount of solids and filter, recover the cyclic phosphoric acid resolving agent, adjust the pH=5.4 of the filtrate with NaOH solution, filter, wash with 15 mL of ice water, and dry. 81.33 g of D-(-)-p-hydroxyphenylglycine was obtained, and...
Embodiment 2
[0036](1) In 400mL isopropanol / water solution (volume ratio is 1:1), add 167g (1mol) of racemized p-hydroxyphenylglycine, 276.41g (1mol) combined cyclic phosphoric acid resolving agent, heat and reflux until the solution is clear , stirred for 1 h, cooled to 50°C, crystals precipitated, filtered, washed the filter cake with 53mL of ice water, and dried to obtain 214.6g of filter cake with a melting point of 231°C-233°C.
[0037] The main resolving agent in the combined cyclic phosphoric acid resolving agent is (-)-CP2, and the auxiliary resolving agent is (-)-CP3, and the molar ratio of the two is 98:2.
[0038] (2) Dissolve the above filter cake in 45mL of phosphoric acid, stir at room temperature for 4h, precipitate a large amount of solid and filter. The cyclic phosphoric acid resolving agent was recovered, and the filtrate was adjusted to pH=5.4 with KOH solution, filtered, washed with 14 mL of ice water, and dried to obtain 79.78 g of D-(-)-p-hydroxyphenylglycine with a y...
Embodiment 3
[0042] (1) In 450mL of methanol / water solution (volume ratio is 1:1), add 167g (1mol) of racemized p-hydroxyphenylglycine, 272.02g (1mol) of combined cyclic phosphoric acid resolving agent, heat and reflux until the solution is clear, and stir After cooling to 50°C for 1h, crystals were precipitated, filtered, washed the filter cake with 50mL of ice water, and dried to obtain 214g of filter cake with a melting point of 226°C-258°C.
[0043] The main resolving agent in the combined cyclic phosphoric acid resolving agent is (-)-CP3, the auxiliary resolving agent is (±)-CP2, and the molar ratio of the two is 99.5:0.5.
[0044] (2) Dissolve the above filter cake in 48mL of 18% dilute sulfuric acid, stir at room temperature for 4h, precipitate a large amount of solid and filter. Reclaim cyclic phosphoric acid resolving agent, filtrate uses Na 2 CO 3 The solution was adjusted to pH=5.4, filtered, washed with 11 mL of ice water, and dried to obtain 79.87 g of D-(-)-p-hydroxyphenylg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com