Polymorphic substances of decitabine and medical compositions
A polymorph, decitabine technology, applied in the directions of drug combinations, antipyretics, sugar derivatives, etc., can solve problems such as increase of related substances, failure to significantly reduce related substances, degradation and destruction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
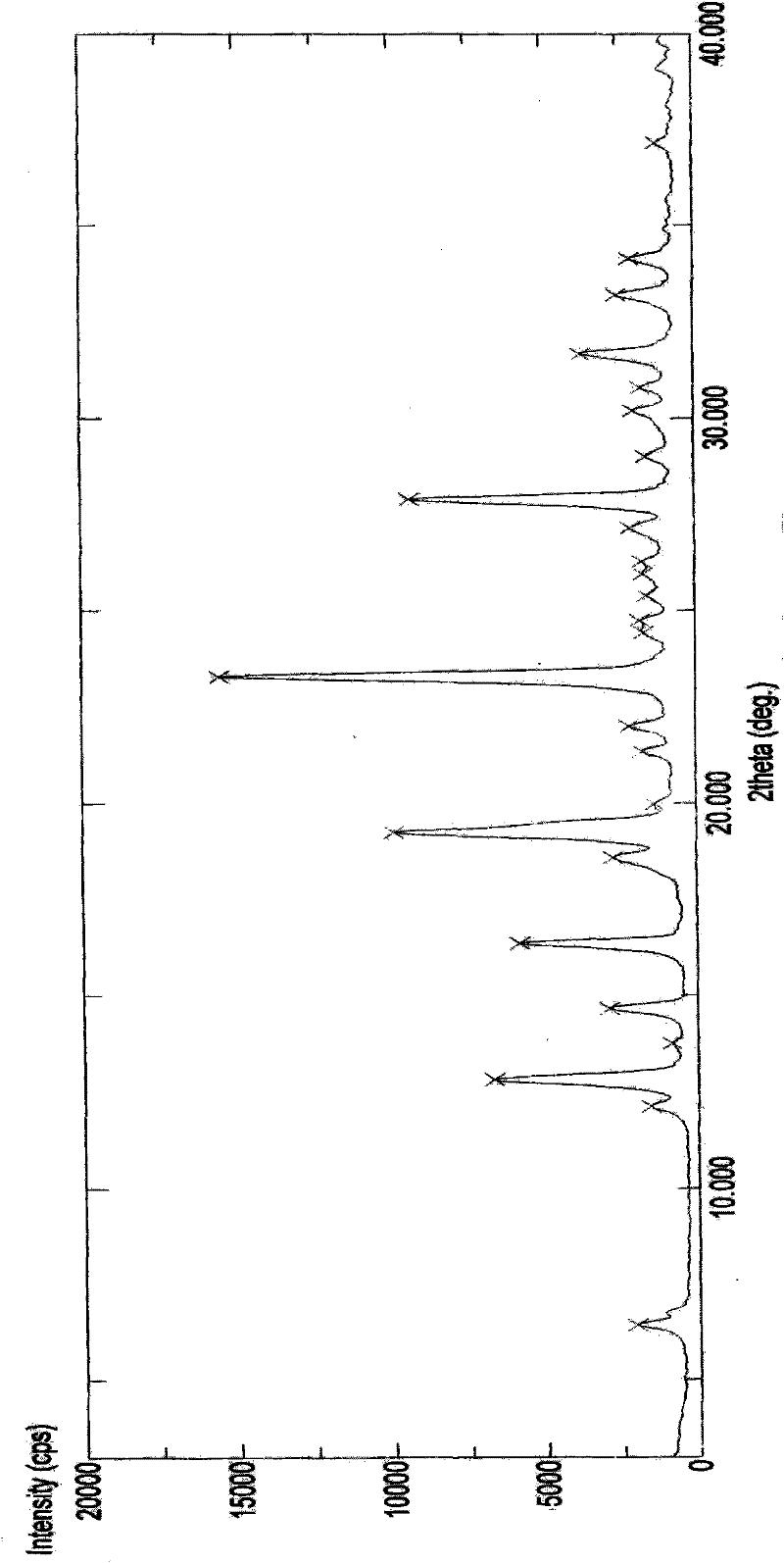
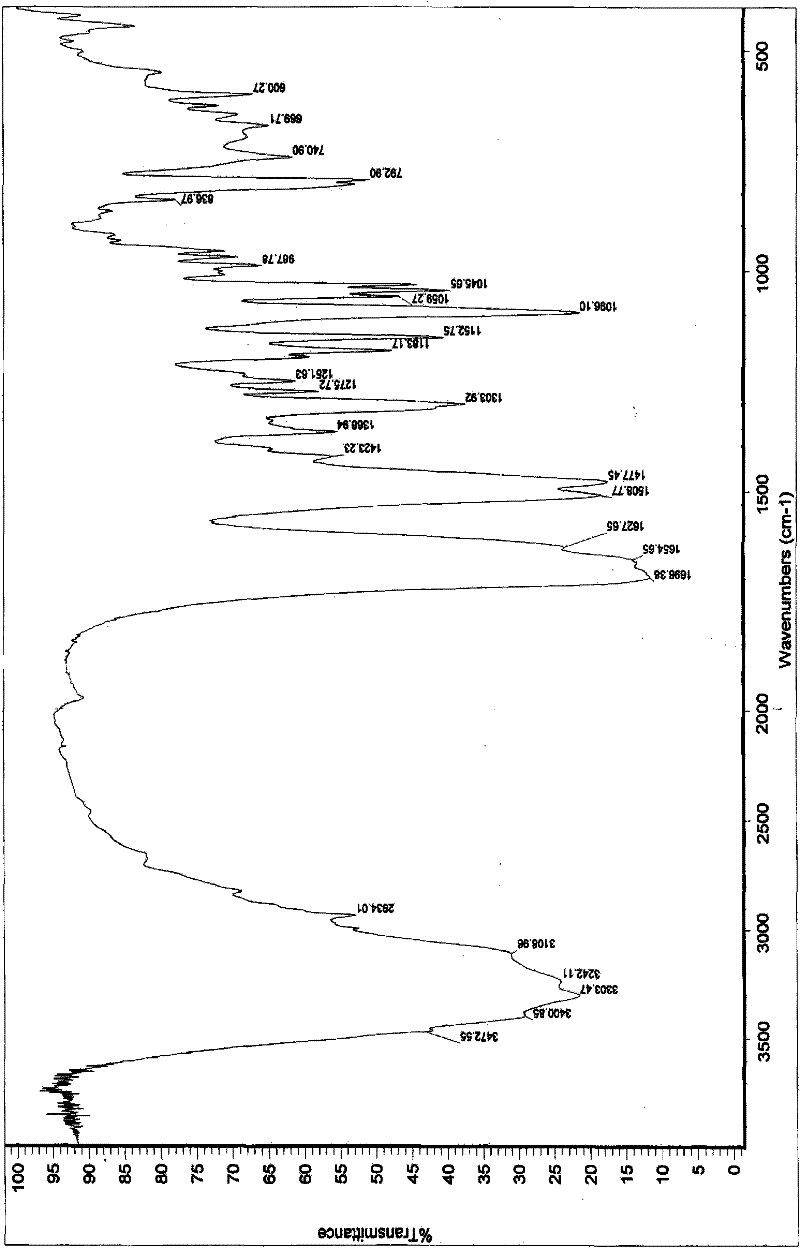
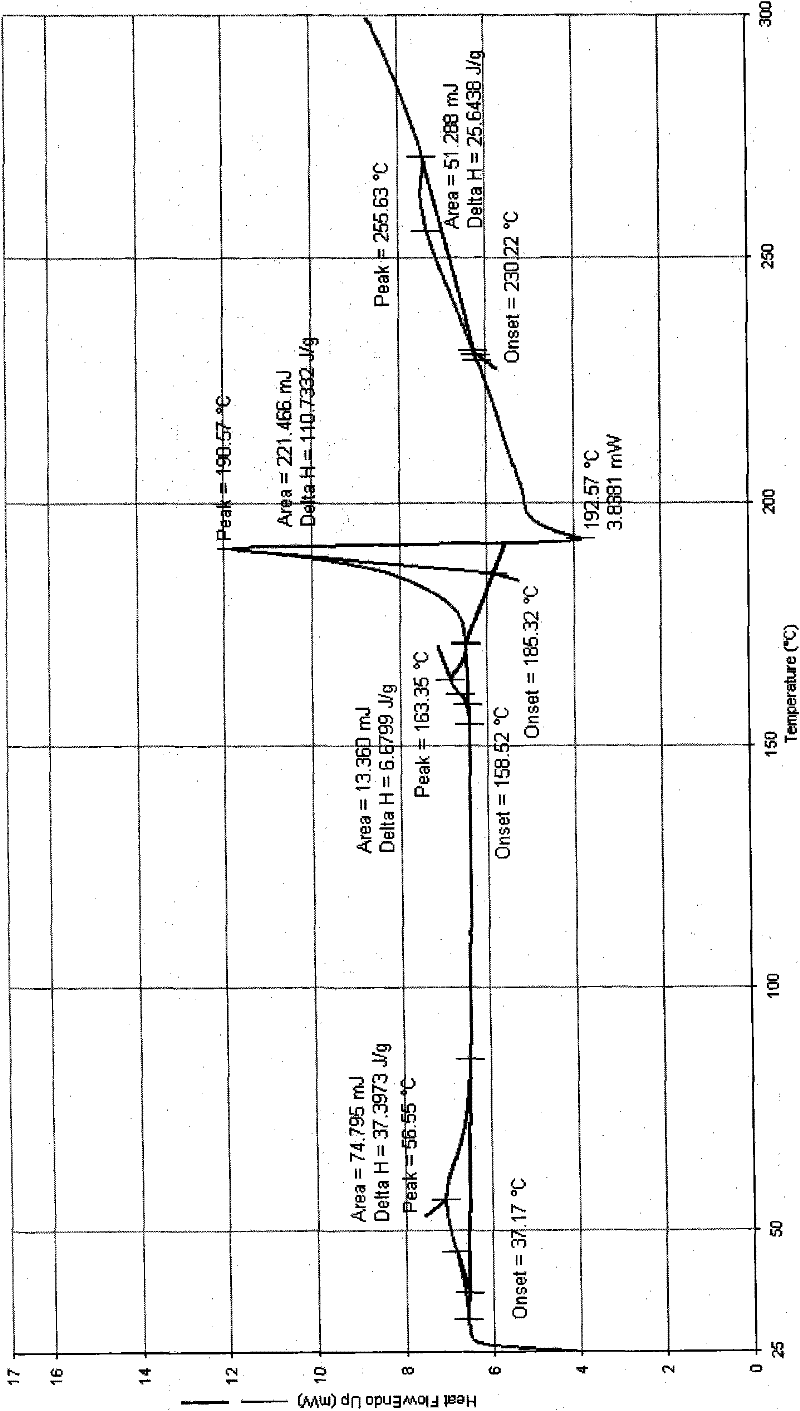
[0162] The preparation of embodiment 1 polymorph I
[0163] plan 1,
[0164] Add 50.0 g of decitabine into the reaction flask, add 100 ml of DMSO, and raise the temperature to about 35° C. while stirring. After dissolving, add 400ml of acetone and 400ml of water, stir for about 10min, then cool down to 0-5°C to grow crystals for 30min. Suction filtration, filter cake rinse with a small amount of acetone. The filter cake was dried under reduced pressure at 50°C and -0.095MPa, and was assisted with phosphorus pentoxide. 31.0 g of white solid was obtained. Yield: 62%.
[0165]
[0166] Scenario 2,
[0167] Add 50.0 g of decitabine into the reaction flask, add 750 ml of DMF, and raise the temperature to about 35° C. while stirring. After dissolving, add 3000ml of water, stir for about 30min and then filter with suction. The filter cake was rinsed with a small amount of acetone. The filter cake was dried under reduced pressure at 50°C and -0.095MPa, and was assisted with...
Embodiment 2
[0172] The preparation of embodiment 2 polymorph II
[0173] plan 1,
[0174] Add 50.0 g of decitabine into the reaction flask, add 750 ml of DMF, and raise the temperature to about 35° C. while stirring. After dissolving, add 1500ml of acetone, stir for about 10 minutes, then cool down to 0-5°C to grow crystals for 30 minutes. Suction filtration, filter cake rinse with acetone. The filter cake was dried under reduced pressure at 50°C and -0.095MPa, and was assisted with phosphorus pentoxide. 40.0 g of white solid was obtained. Yield: 80%.
[0175]
[0176] Scenario 2,
[0177] Add 50.0 g of decitabine into the reaction flask, add 750 ml of DMF, and raise the temperature to about 35° C. while stirring. After dissolving, add 1500ml of ethyl acetate, stir for about 10 minutes, then cool down to 0-5°C to grow crystals for 30 minutes. Suction filtration, filter cake rinse with ethyl acetate. The filter cake was dried under reduced pressure at 50°C and -0.095MPa, and was...
Embodiment 3
[0186] The preparation technology of embodiment 3 decitabine aseptic powder preparation:
[0187] Dissolve 50 grams of decitabine raw material in 250ml of dimethylformamide or dimethyl sulfoxide solution, heat to 40°C under stirring to dissolve, add activated carbon and stir for ten minutes, filter through a 0.45um microporous membrane Filtration, the filtrate is then filtered with a 0.22um microporous membrane, and the filtered filtrate is transferred to a sterile clean area;
[0188] Add about 2000ml of water or acetone or a mixture of water and acetone, add activated carbon and stir for ten minutes, filter through a 0.45um microporous membrane, then filter through a 0.22um microporous membrane, and transfer the filtered filtrate to a sterile Clean area;
[0189] Water or an organic solvent or a sterile mixed solution of water and an organic solvent is added dropwise to the above-mentioned sterile solution of decitabine dimethylformamide or dimethyl sulfoxide in about 15 mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com