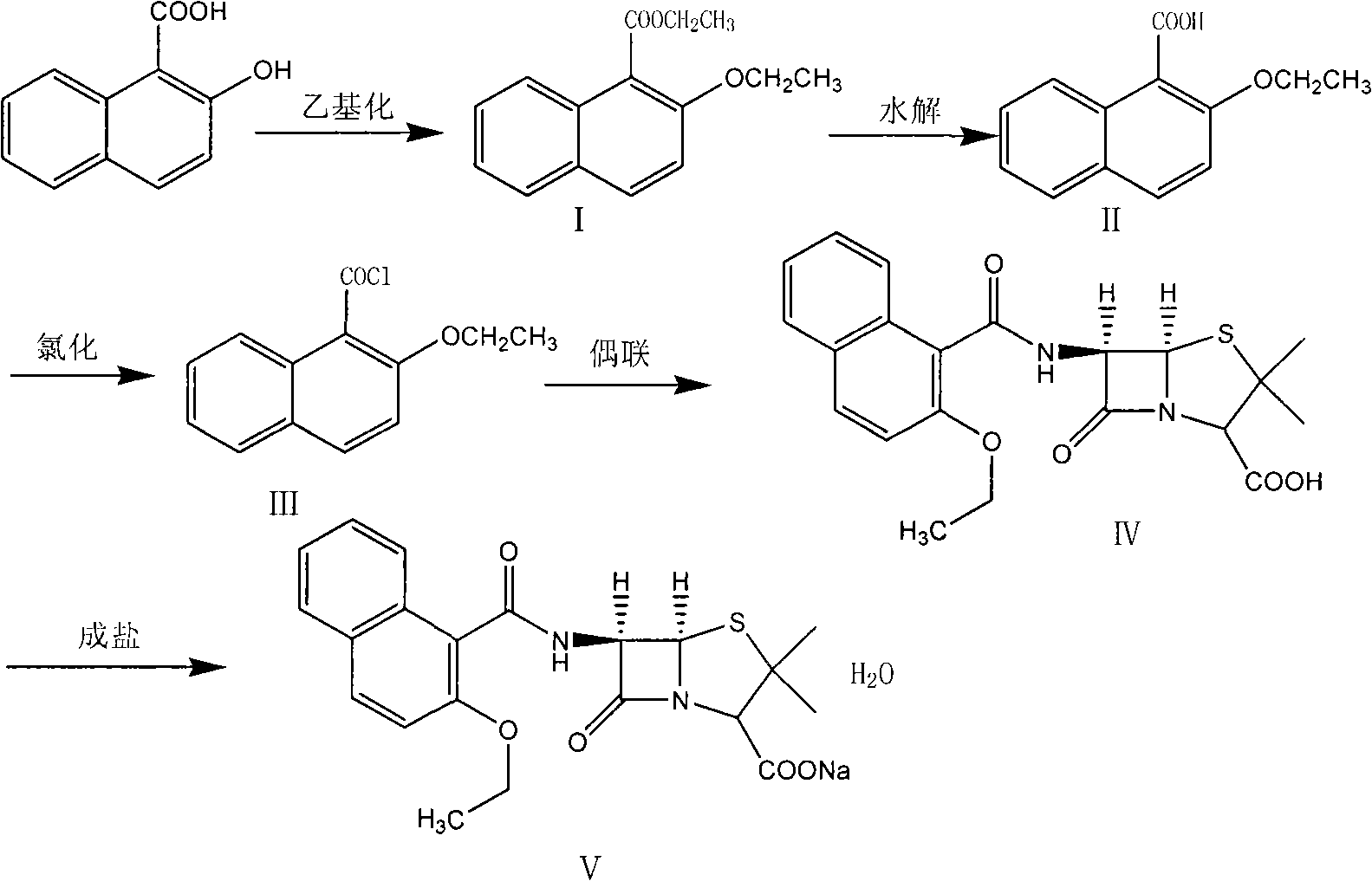
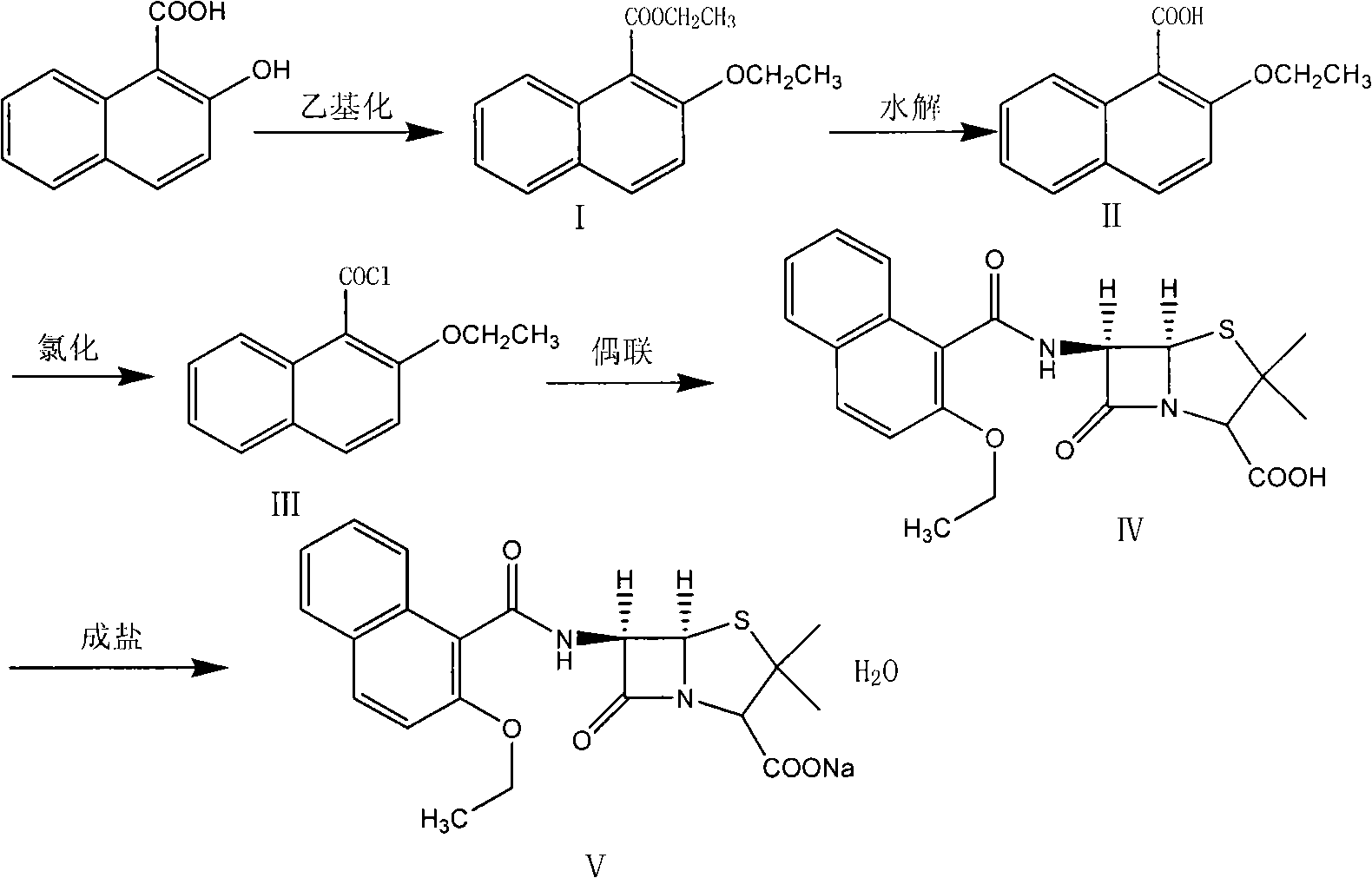
Synthesizing method of nafcillin sodium-hydrate
A technology of nafcillin sodium monohydrate and synthesis method, which is applied in the field of medicine and can solve problems such as the instability of nafcillin sodium
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 38Kg of DMF and 18.82Kg of 2-hydroxy-1-naphthoic acid into a 200L reactor, stir to dissolve, control the temperature at 20-30°C, add 8.8Kg of sodium hydroxide, and continue stirring for 30 minutes after adding, to obtain a gray viscous liquid. Add 38.7 kg of diethyl sulfate dropwise. After the addition, a clear brown liquid was obtained. Continue stirring for 5 h. Under cooling in an ice-water bath, add 80 kg of 10% NaOH aqueous solution, stir for 30 min, and separate into layers. The aqueous layer is extracted twice with 35 kg of ether and combined. The ether layer and the oil layer were distilled off to obtain 17.1Kg of brown oil intermediate (I) ethyl 2-ethoxy-1-naphthoate.
Embodiment 2
[0017] Add 38Kg of DMF and 18.82Kg of 2-hydroxy-1-naphthoic acid into a 200L reactor, stir to dissolve, control the temperature at 20-30°C, add 8.8Kg of sodium hydroxide, and continue stirring for 30 minutes after adding, to obtain a gray viscous liquid. Add 16.2 kg of bromoethane dropwise, and after the addition, a clear brown liquid is obtained. Continue to stir for 5 h, add 80 kg of 10% NaOH aqueous solution under cooling in an ice-water bath, stir for 30 min, and separate into layers. The aqueous layer is extracted twice with 35 kg of ether, and the ether is combined. layer and oil layer, diethyl ether was distilled off to obtain 14.2Kg of brown oil intermediate (I) ethyl 2-ethoxy-1-naphthoate.
Embodiment 3
[0019] Add 17kg of intermediate (I), 15.7kg of potassium hydroxide, and 63kg of 95% ethanol in a 200L reactor, reflux for 4h, concentrate under reduced pressure to obtain a solid, add water to dissolve the solid, and wash the above solution twice with dichloromethane; Add 10% dilute hydrochloric acid to the layer dropwise to pH 2.0-3.0, stir at room temperature for 1 hour, filter, rinse the filter cake with a large amount of water until the filtrate is neutral; dry to obtain a brown powder
[0020] (II) 14.1 kg of 2-ethoxy-1-naphthoic acid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com