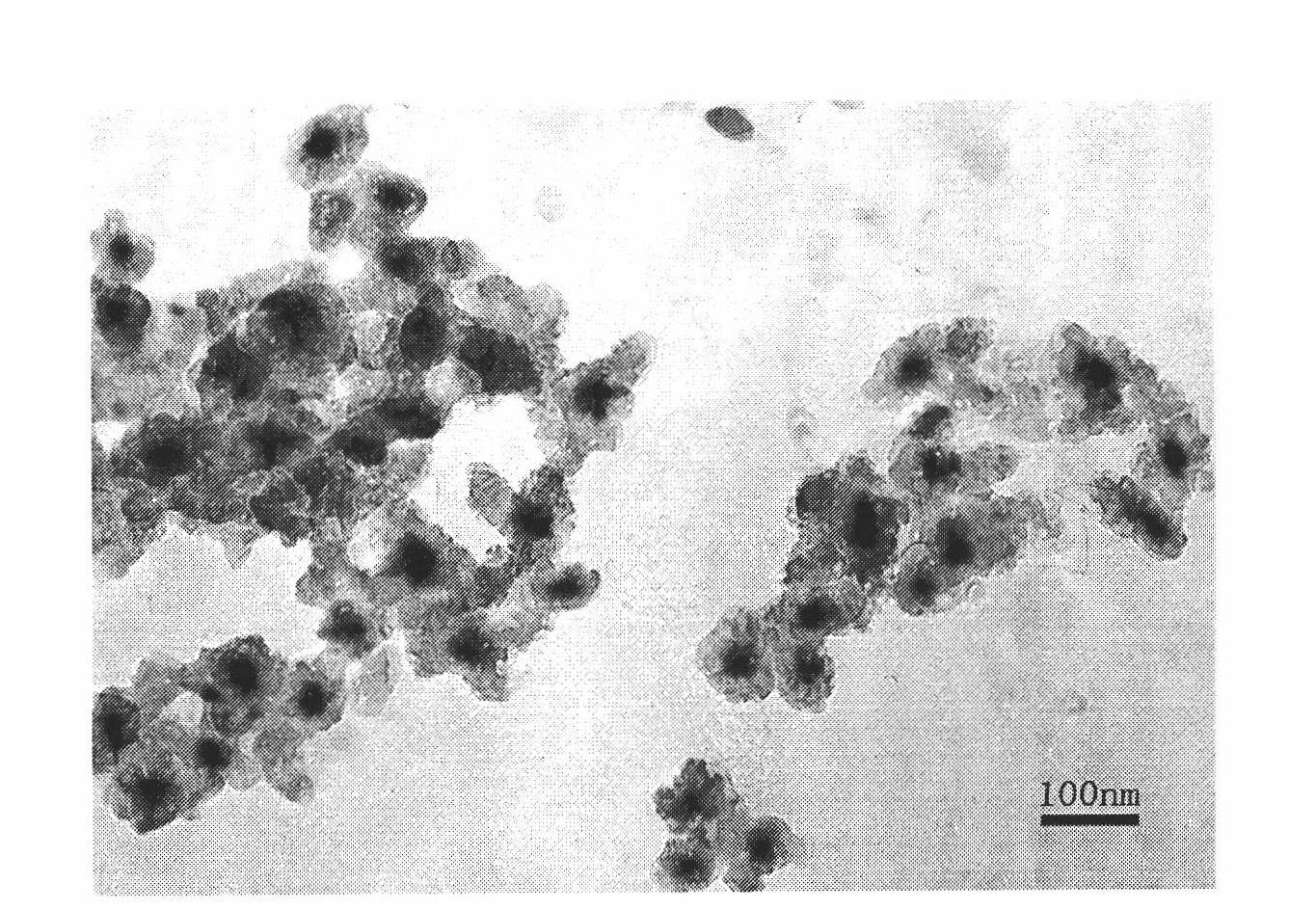
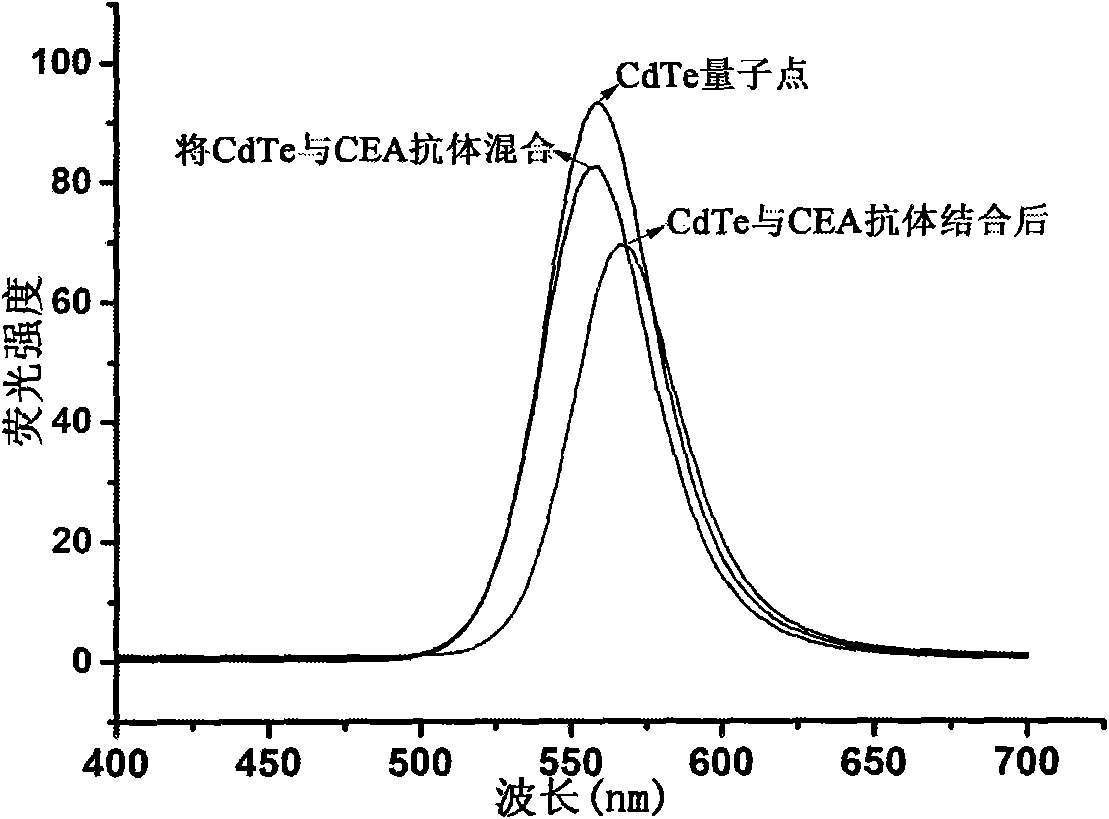
Luminescent and magnetic nano material-based method for detecting carcinoembryonic antigen
A magnetic nano, fluorescent nano technology, applied in fluorescence/phosphorescence, analytical materials, material excitation analysis, etc., can solve the problems of poor photostability, short luminescence time, long emission spectrum, etc., to achieve simplified detection methods and high sensitivity , The effect of covalent binding and stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Fe 3 o 4 / SiO 2 Preparation of core-shell nanoparticles:
[0041] 1. Weigh 1.08g of FeCl 3 ·6H 2 O and 0.555g FeSO 4 ·7H 2 O, dissolved in 20ml ultrapure water, vacuumed for 20min, N 2 Saturation for 20 minutes; add excess ammonia water to it to obtain a black precipitate, combined with magnetic separation, wash with ultrapure water several times.
[0042] 2. Mix 7 mg of the product from step 1 with 2.3 mL of absolute ethanol, 2.5 mL of deionized water and 0.7 mg of sodium oleate, stir with a magnet and add 10 μL of GMPS.
[0043] 3. Sonicate the mixture in step 2 for 15 minutes, add 100 μL of TEOS, stir for 1 hour, add 10.2 mL of 28% ammonia water dropwise, and stir for 20 hours to obtain Fe 3 o 4 / SiO 2 Core-shell nanoparticles, combined with magnetic separation, were washed several times with ultrapure water.
[0044] (2) Preparation of CdTe quantum dots:
[0045] 1. Vacuumize 40ml of buffer solution containing 15mM boric acid and 15mM sodium citrate ...
Embodiment 2
[0062] Detect the content of CEA in the patient's serum:
[0063] 1. Take 1ml of serum and mix with 100μl of "Fe 3 o 4 / SiO 2 - The first antibody" was mixed and reacted at room temperature for 30 minutes;
[0064] 2. Combining with magnetic separation, wash each microcentrifuge tube sample in step 1 with PBS buffer solution of pH 7.4 twice, and then dissolve to 1.0ml, then add 200μl of "CdTe-secondary antibody" complex respectively, and store at room temperature React for 30 minutes;
[0065] 3. Combined with magnetic separation, carefully wash twice with PBS buffer solution with pH 7.4, use the buffer solution to make up to 0.5ml, and measure fluorescence;
[0066] 4. Bring the obtained results into the working equation Y=A(X-B), calculate the content of CEA in the serum, and compare the results with the results of immunofluorescence detection, see Table 1.
[0067] Table 1 Detection method of the present invention and immunofluorescence detection result contrast
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com