Polymorphs of abiraterone acetate and preparation method thereof
A technology of abiraterone acetate and abiraterone acetate, which is applied to the polymorphic form of abiraterone acetate and its preparation field, can solve the problem of different binding force of crystal particles, influence on drug dissolution rate, influence on drug fluidity and pressure. Issues such as tablet hardness, tablet weight variation, content uniformity, and physical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
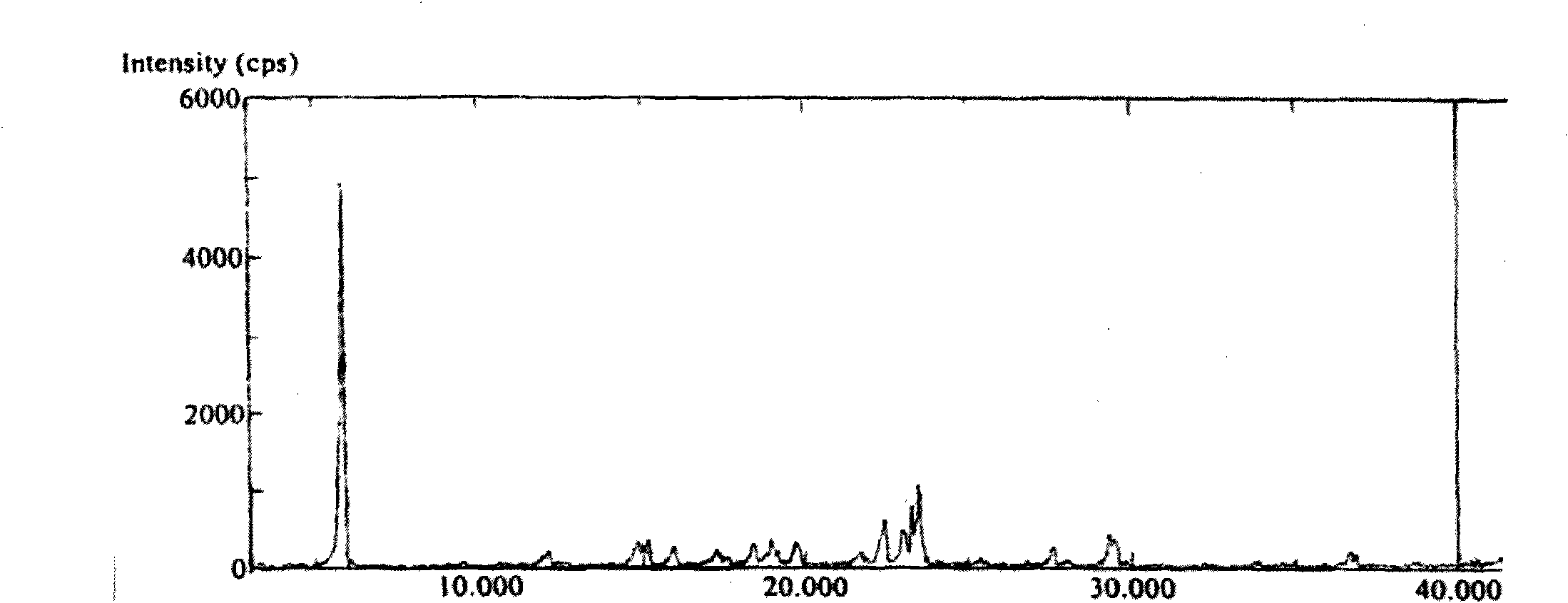
[0041] The preparation of embodiment 1 abiraterone acetate polymorph A
[0042] Add 30g of pure abiraterone acetate obtained through column purification into 140ml of ethyl acetate, stir and dissolve at room temperature, slowly add 700ml of petroleum ether after dissolution, concentrate the whole to 280ml, place at room temperature for crystallization, filter, and oven at 50°C After drying for 4 hours, 15.2 g of white crystals were obtained, yield: 76%.
[0043] HPLC test purity 99.9239%, the largest single impurity 0.0761%,
[0044] Moisture 0.00%
[0045] DSC melting point: 144.7-145.7°C
[0046] Specific rotation: -38.0 (C=1.0g / 100ml, ethanol)
Embodiment 2
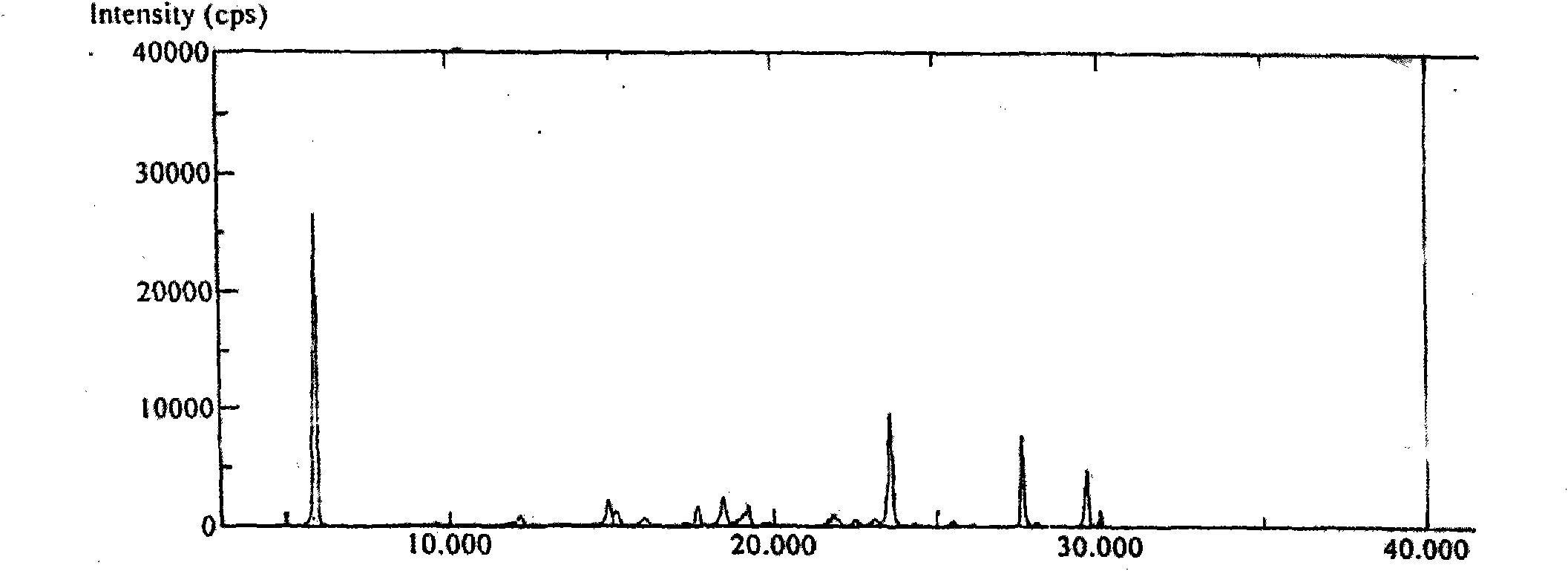
[0047] Embodiment 2 Preparation of Abiraterone Acetate Polymorph A
[0048] Add 30 g of pure abiraterone acetate obtained through column purification into 180 mL of ethanol / n-hexane with a volume ratio of 1:9, heat and stir to dissolve, filter while hot after complete dissolution, place at room temperature for crystallization, filter, 50°C Oven-dried for 4 hours to obtain 12.5 g of white crystals, yield: 62.5%.
[0049] HPLC test purity 99.9812%, the largest single impurity 0.0155%,
[0050] Moisture 0.00%
[0051] DSC melting point: 144.9-146.0°C
[0052] Specific rotation: -39.98 (C=1.0g / 100ml, ethanol)
Embodiment 3
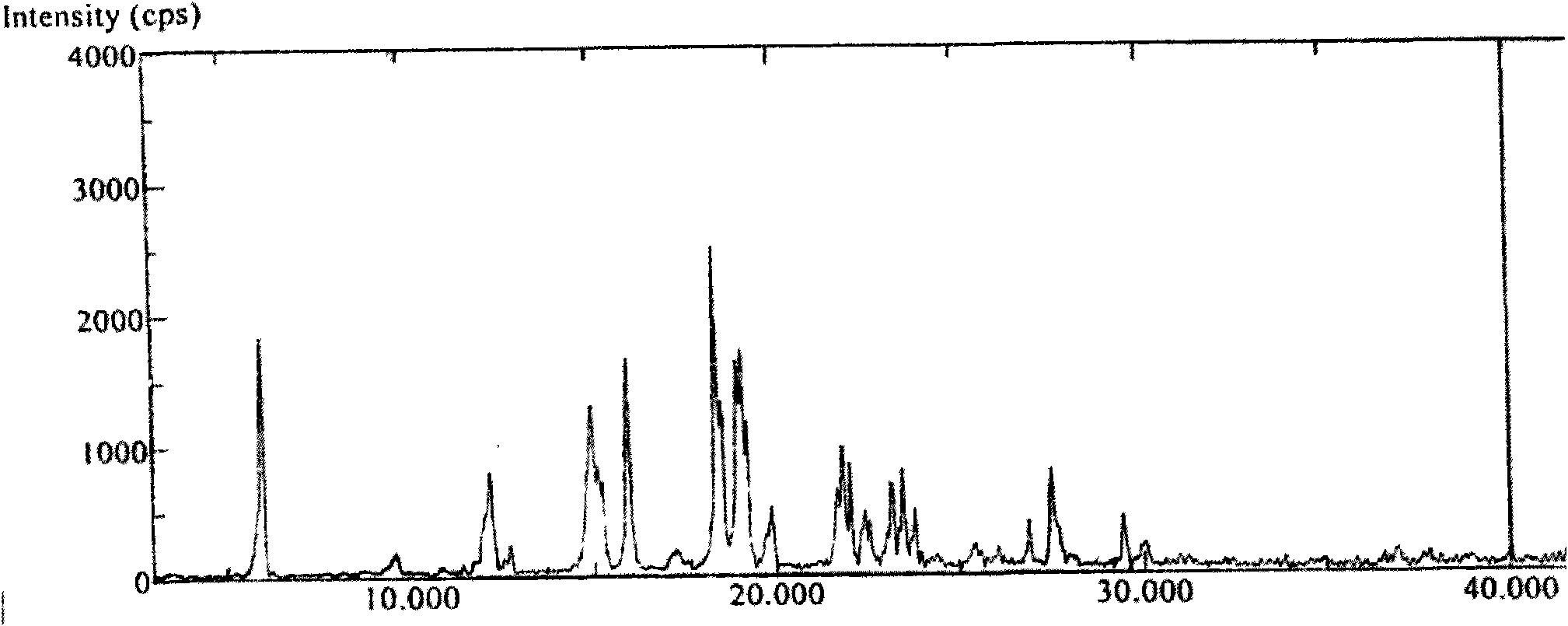
[0053] Embodiment 3 Preparation of Abiraterone Acetate Polymorph A
[0054] Add 30 g of pure abiraterone acetate obtained through column purification into 140 mL of ethanol / water with a volume ratio of 1:9, heat and stir to dissolve, filter while hot after complete dissolution, place crystallization at room temperature, filter, and dry in an oven at 70°C After drying for 2 hours, 16.5 g of white crystals were obtained, yield: 62.5%.
[0055] HPLC test purity 99.7903%, the largest single impurity 0.1065%,
[0056] Moisture: 0.00%
[0057] DSC melting point: 144.5-145.1°C
[0058] Specific rotation: -38.91 (C=1.0g / 100ml, ethanol)
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com