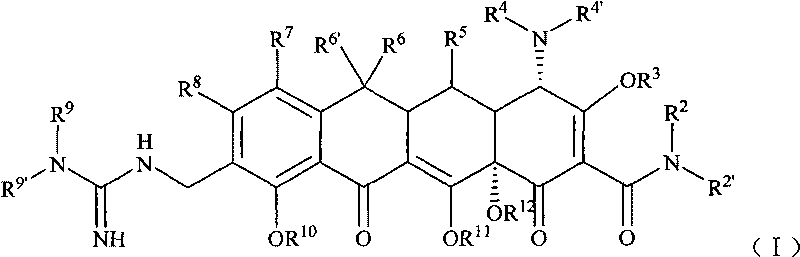
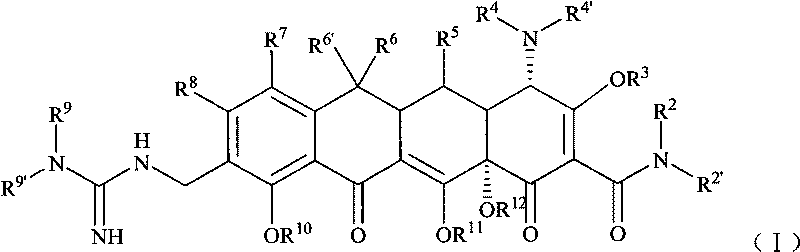
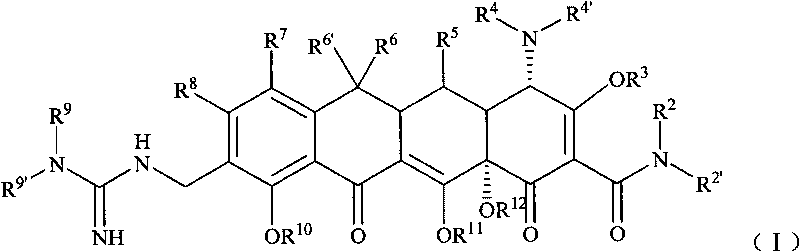
Guanidyl substitutional tetracycline derivative
An alkyl and amino technology, which is used in the field of anti-infective drugs, preparation, treatment and/or prevention of tetracycline-sensitive diseases, and can solve problems such as unsatisfactory activity of Gram-negative bacteria, pain of patients, inconvenient medication, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 [S-(4α, 12aα)]-9-[N'-tert-butylguanidino]methyl-4,7-bis(dimethylamino)-1,4,4a,5,5a, 6, 11, 12a-octahydro -3,10,12,12a-tetrahydroxyl-1,11-dioxo 2-naphthacene carboxamide (compound 1) preparation
[0100] Step 1 [S-(4α,12aα)]-9-aminomethyl-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro - Preparation of 3,10,12,12a-tetrahydroxyl-1,11-dioxo-2-naphthacenecarboxamide
[0101] Put [S-(4α,12aα)]-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10 into the reaction flask, 12,12a-Tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide 4.6g (10mmol), add 100ml of trifluoroacetic acid, stir to dissolve, then add 3.6g (20mmol) hydroxymethyl-benzyl carbamate ester (HMBC), stirred at room temperature for 2 d, after the reaction was completed, the reaction mixture was filtered, and the filtrate was concentrated under reduced pressure to remove trifluoroacetic acid. Can be purified by preparative HPLC. 2.0 g of solid compound was obtained, yield: 41%.
[010...
Embodiment 2
[0108] Example 2 [S-(4α, 12aα)]-9-[N'-(N,N-dimethyl)guanidino]methyl-4,7-bis(dimethylamino)-1,4, 4a, 5, 5a, 6, 11, 12a- Preparation of octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide (compound 2)
[0109] Referring to Step 2 of Example 1, cast [S-(4α, 12aα)]-9-aminomethyl-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11, 12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide 12.2g (25mmol), N,N-dimethylcyanamide 3.5g (50mmol ). 6.9 g of the target compound was obtained, yield: 50.0%.
[0110] Molecular formula: C 27 h 36 N 6 o 7 Molecular weight: 556.61 Mass spectrum (m / e): 557 (M+1)
[0111] Elemental analysis: theoretical value: C, 58.26%; H, 6.52%; N, 15.10%
[0112] Found: C, 58.15%; H, 6.65%; N, 15.01%
[0113] 1 H-NMR (600MHz, CDCl 3 ): δ1.45(t, 1H), 1.77(t, 1H), 2.12(s, 1H), 2.28(s, 1H), 2.32(t, 1H), 2.34(s, 6H), 2.38(m, 1H), 2.45(s, 6H), 2.48(d, 1H), 2.71(d, 1H), 2.83(s, 6H), 3.27(d, 1H), 4.03(s, 2H), 5.14(s...
Embodiment 3
[0114] Example 3 [S-(4α, 12aα)]-9-[N'-(N,N-diethyl)guanidino]methyl-4,7-bis(dimethylamino)-1,4, 4a, 5, 5a, 6, 11, 12a- Preparation of octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide (compound 3)
[0115] Referring to Step 2 of Example 1, cast [S-(4α, 12aα)]-9-aminomethyl-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11, 12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-tetracenecarboxamide 12.2g (25mmol), N,N-diethylcyanamide 4.9g (50mmol ). 7.4 g of the target compound was obtained, yield: 50.5%.
[0116] Molecular formula: C 29 h 40 N 6 o 7 Molecular weight: 584.66 Mass spectrum (m / e): 585 (M+1)
[0117] Elemental analysis: theoretical value: C, 59.57%; H, 6.90%; N, 14.37%
[0118] Found: C, 59.47%; H, 7.00%; N, 14.25%
[0119] 1 H-NMR (600MHz, CDCl 3 ): δ1.03(t, 6H), 1.46(t, 1H), 1.75(t, 1H), 2.08(s, 1H), 2.26(s, 1H), 2.29(s, 6H), 2.31(t, 1H), 2.36(m, 1H), 2.46(d, 1H), 2.57(q, 4H), 2.70(d, 1H), 2.83(s, 6H), 3.25(d, 1H), 4.02(s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com