Combined test reagent card for cytomegalovirus and rubella virus
A technology for cytomegalovirus and rubella virus, applied in the field of joint detection cards, can solve the problems of many factors affecting IgM antibody detection, prone to false positives, short maintenance time, etc., achieve high accuracy, broad application prospects, and reduce procedures Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
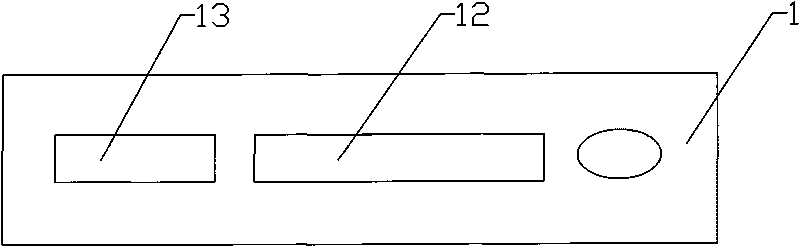
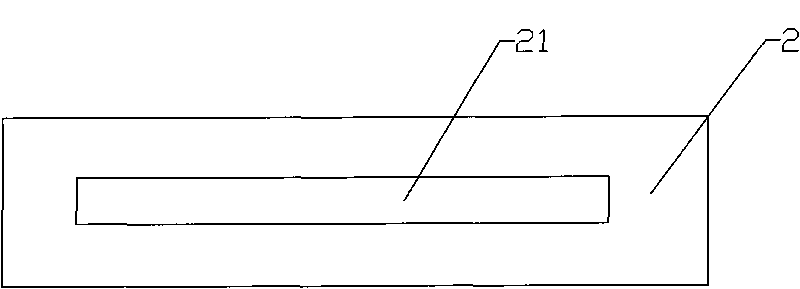
[0043] Example 1: Such as figure 1 with figure 2 As shown, a cytomegalovirus and rubella virus combined detection reagent test card includes a card cover 1, a card bottom 2 and a test strip 3. The card cover 1 is provided with a sample hole 11, an observation hole 12 and an installation marking hole 13 , The card bottom 2 is provided with a groove 21 in which the test strip 3 is placed. The test sample application area 31 on the test strip 3 corresponds to the sample injection hole 11 on the card cover 1; the gold label area 32 and the nitrocellulose area 33 on the test strip 3 correspond to the observation hole 12 on the card cover 1. correspond.
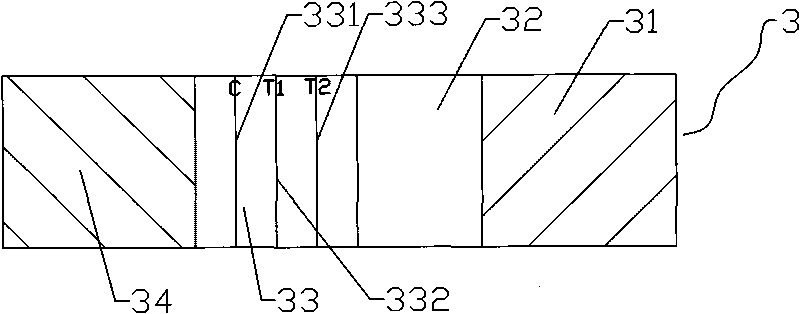
[0044] Figure and Figure 4 As shown, the test strip 3 is provided with a test sample area 31, a gold label area 32, a nitrocellulose membrane area 33, and a water absorption area 34 in sequence. The test sample area 31 is a glass fiber layer; the gold label area 32 is provided with a test strip 3 On the upper surface of the colloi...
Embodiment 2
[0071] Embodiment 2: A cytomegalovirus and rubella virus combined detection reagent test card, the structure of which is the same as that of embodiment 1.
[0072] A method for preparing a cytomegalovirus and rubella virus combined detection reagent detection card is assembled from the above-mentioned card cover 1, card bottom 2 and test strip 3, and the test strip 3 is sequentially provided with a detection sample area 31 and a gold label area 32. The nitrocellulose membrane area 33 and the water absorption area 34. The gold label area 32 is provided with a colloidal gold layer 321. The colloidal gold layer 321 consists of chloroauric acid solution, trisodium citrate solution, potassium carbonate solution and anti-human IgG monoclonal antibody The solution is prepared.
[0073] The preparation process of the colloidal gold layer 321 is as follows:
[0074] A. Take 100ml of 4% chloroauric acid aqueous solution, heat it to boiling, add 0.8ml 0.5% trisodium citrate solution, mix quick...
Embodiment 3
[0085] Embodiment 3: A cytomegalovirus and rubella virus combined detection reagent test card, the structure of which is the same as that of embodiment 1.
[0086] A method for preparing a cytomegalovirus and rubella virus combined detection reagent detection card is assembled from the above-mentioned card cover 1, card bottom 2 and test strip 3, and the test strip 3 is sequentially provided with a detection sample area 31 and a gold label area 32. The nitrocellulose membrane area 33 and the water absorption area 34. The gold label area 32 is provided with a colloidal gold layer 321. The colloidal gold layer 321 consists of chloroauric acid solution, trisodium citrate solution, potassium carbonate solution and anti-human IgG monoclonal antibody The solution is prepared.
[0087] The preparation process of the colloidal gold layer 321 is as follows:
[0088] A. Take 100ml of 2% chloroauric acid aqueous solution, heat to boiling, add 0.8ml of 0.8% trisodium citrate solution, mix quick...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com