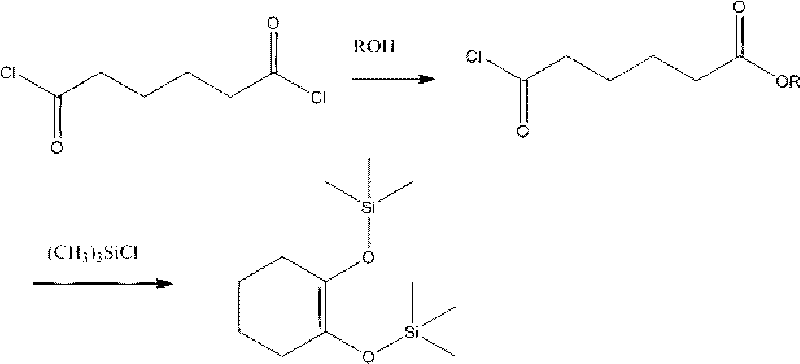
Preparation method of 1,2-bi-trimethylsilyloxy cyclohexene
A technology of trimethylsiloxane and trimethylchlorosilane, which is applied in the field of 1, can solve the problems of large amount of trimethylchlorosilane, unsuitable for industrialization, high production cost, etc., and achieves less side reactions and less destructiveness , cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 500ml three-necked flask equipped with mechanical stirring and a thermometer, put 186g (1.0mol) of adipic acid dichloride and 150ml of methylene chloride, stir and cool to 0°C, and add the mixture of methanol and methylene chloride dropwise at a constant speed for 1 hour[ Methanol 34.5g (1.078mol), dichloromethane 50ml], continue to react at 0 ℃ for 1 hour after dropping, heat up to 15-30 ℃ and react for 1 hour, after the reaction is completed, dichloromethane is distilled under reduced pressure, and the residue is reduced Pressure rectification, collection 107-108 ℃ (10mmHg) is monomethyl adipate monoacyl chloride, weight 164.0g, content 98.0% (GC) yield 90.0%.
Embodiment 2
[0030] Put 186g (1.0mol) of adipic dichloride and 150ml of carbon tetrachloride into a 500ml three-necked flask equipped with mechanical stirring and a thermometer, stir and cool to 0°C, and add anhydrous ethanol and tetrachloride dropwise at a constant speed for 1 hour. Carbon mixture [absolute ethanol 49.5g (1.075mol), carbon tetrachloride 70ml], after dripping, continue to react at 0°C for 1 hour, raise the temperature to 25-30°C and react for 1 hour, after the reaction is completed, tetrachloride is distilled off under reduced pressure. Carbon chloride, rectification under reduced pressure on the residue, collected at 115-117°C (10mmHg) as monoethyl adipate monoacyl chloride, weight 178.0g, content 98.0% (GC) yield 90.5%.
Embodiment 3
[0032] Put 186g (1.0mol) of adipic acid dichloride and 150ml of trichlorethylene into a 500ml three-necked bottle equipped with mechanical stirring and a thermometer, stir and cool to 0°C, and add anhydrous ethanol and trichlorethylene dropwise at a constant speed for 1 hour. Mixture [absolute ethanol 49.5g (1.075mol), trichlorethylene 70ml], continue to react at 0°C for 1 hour after dripping, raise the temperature to 25-50°C for 1 hour, after the reaction is complete, distill off trichlorethylene under reduced pressure, The residue was rectified under reduced pressure and collected at 115-117°C (10mmHg) as ethyl adipate monoacyl chloride, with a weight of 177.8g, a content of 98.0% (GC) and a yield of 90.4%.
[0033] The solvent in above-mentioned embodiment 1-3 also can replace with trichloromethane, tetrachloroethylene, toluene, ethylbenzene, xylene, isopropylbenzene, trimethylbenzene, normal hexane, sherwood oil, hexanaphthene or methylcyclohexane The alcohol used in the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com