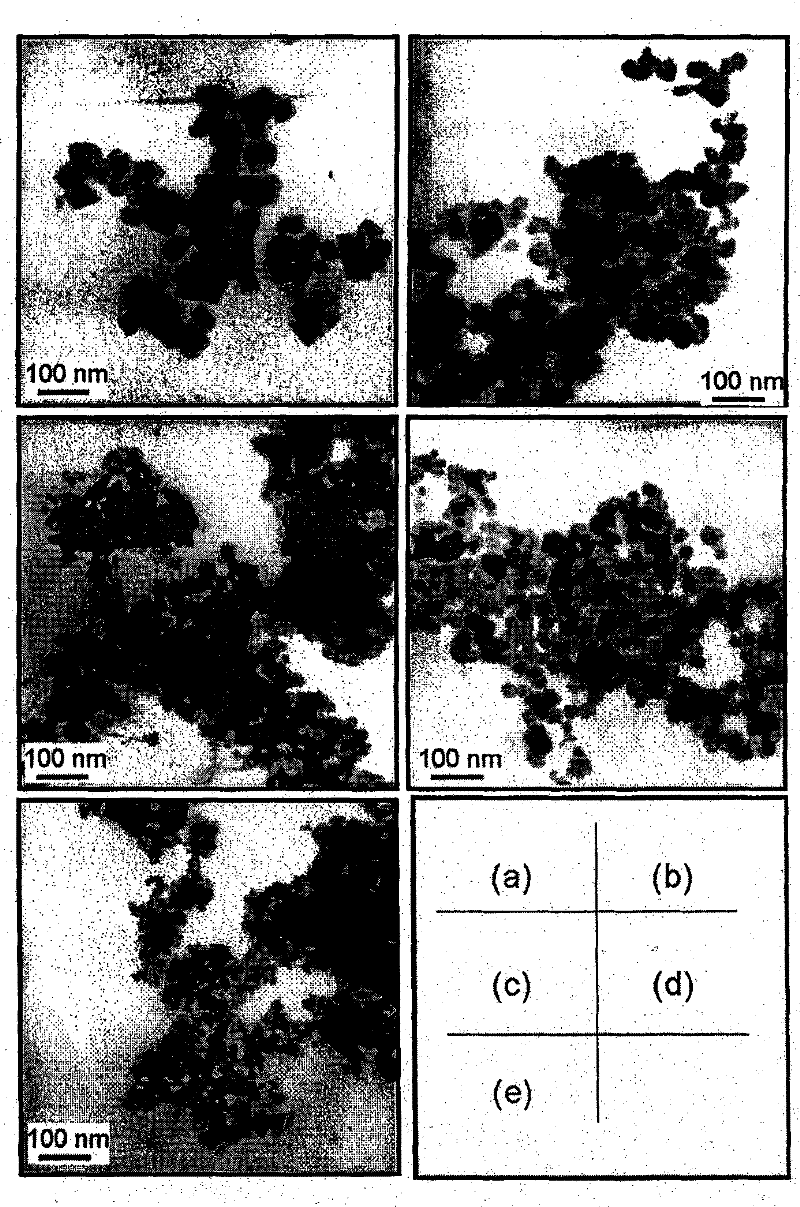
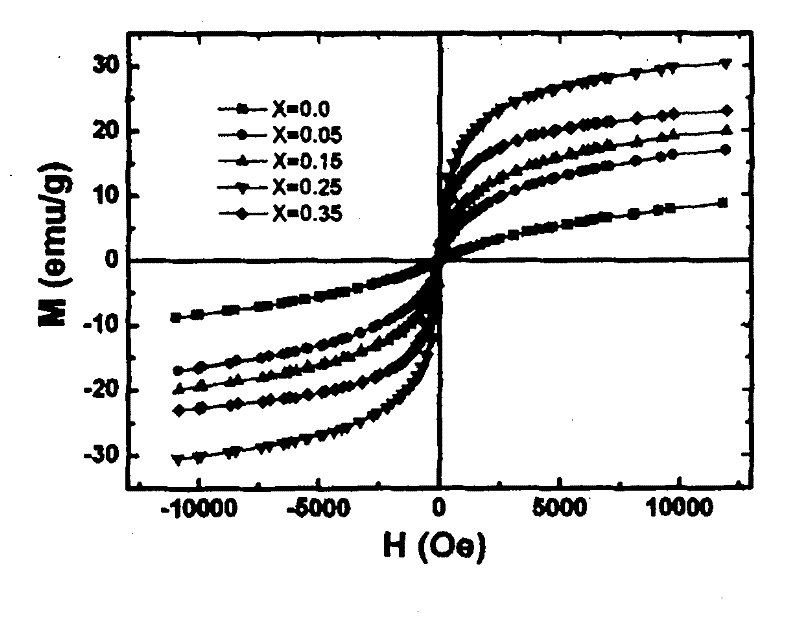
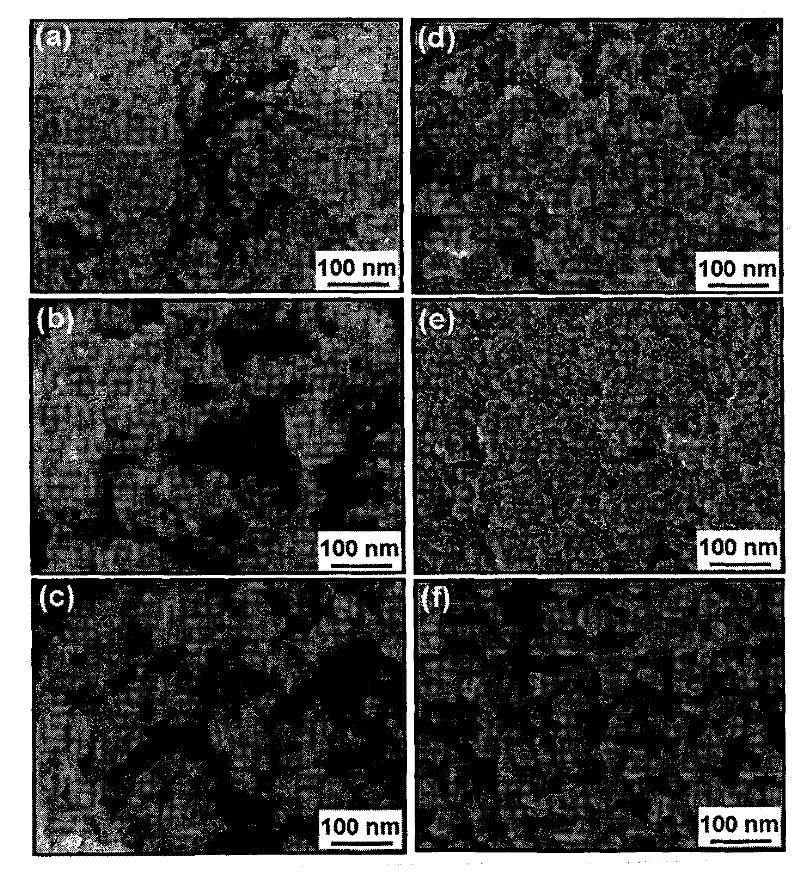
Preparation method of Mn-Zn ferrite cobalt-doped nano material
A manganese-zinc ferrite and cobalt nanotechnology, which is applied in the nanometer field, can solve the problems of decreased magnetic properties of materials, high purity of raw materials, and difficult shape control, and achieve the effects of improving interface properties, simple experimental equipment, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Under normal temperature and pressure, press Mn 0.5 Zn 0.5 Fe 2 O 4 The molar ratio of Mn, Zn, and Fe, weigh 1.9791g of MnCl 2 ·4H 2 O, 2.8756g of ZnSO 4 ·7H 2 O and 10.8116g FeCl 3 ·6H 2 Put O into the beaker, add deionized water and stir to completely dissolve ferric chloride, zinc sulfate and manganese chloride, then pour the dissolved mixture into a 50mL volumetric flask, and then rinse the remaining mixture in the beaker with deionized water Pour it into a volumetric flask, bring it to a constant volume of 50 mL and shake it up to prepare a manganese zinc iron salt solution with an ion concentration of 1.5 mol / L. Weigh 4.6133g cetyltrimethylammonium bromide (CTAB), 9.2267g n-butanol, 30ml isooctane into a three-necked flask, and add 4.558g salt solution with an ion concentration of 1.5mol / L to the flask, Mix and stir until CTAB is completely dissolved and the R value is 20 (molar ratio of water phase to surfactant R=n[H 2 O] / n[CTAB] is 15-25) clear and tra...
Embodiment 2
[0040] Under normal temperature and pressure, press Mn 0.5 Zn 0.45 Co 0.05 Fe 2 O 4 The molar ratio of Mn, Zn, Co, and Fe, weigh 1.9791g of MnCl 2 ·4H 2 O, 2.5880g ZnSO 4 ·7H 2 O, 10.8116g FeCl 3 ·6H 2 O and 0.2380g of CoCl 2 ·6H 2 Put O in the beaker, add deionized water and stir to completely dissolve ferric chloride, zinc sulfate, manganese chloride and cobalt chloride, then pour the dissolved mixture into a 50mL volumetric flask, and then rinse the beaker with deionized water. Put it into a volumetric flask, bring it to a constant volume of 50 mL, shake well, and prepare a manganese zinc cobalt iron salt solution with an ion concentration of 1.5 mol / L. According to CTAB: n-butanol = 1:2, (CTAB + n-butanol): isooctane = 2:3, weigh 4.6133g CTAB, 9.2267g n-butanol, 30ml isooctane into a three-necked flask, add 4.558g The salt solution with ion concentration of 1.5mol / L is mixed and stirred until CTAB is completely dissolved and the R value is 20 (molar ratio of water phase to s...
Embodiment 3
[0042] Under normal temperature and pressure, press Mn 0.5 Zn 0.35 Co 0.15 Fe 2 O 4 The molar ratio of Mn, Zn, Co, and Fe, weigh 1.9791g of MnCl 2 ·4H 2 O, 2.01292g ZnSO 4 ·7H 2 O, 10.8116g FeCl 3 ·6H 2 O and 0.71385g of CoCl 2 ·6H 2 Put O in the beaker, add deionized water and stir to completely dissolve ferric chloride, zinc sulfate, manganese chloride and cobalt chloride, then pour the dissolved mixture into a 50mL volumetric flask, and then rinse the beaker with deionized water. Put it into a volumetric flask, bring it to a constant volume of 50 mL, shake well, and prepare a manganese zinc cobalt iron salt solution with an ion concentration of 1.5 mol / L. According to CTAB: n-butanol = 1:2, (CTAB + n-butanol): isooctane = 2:3, weigh 4.6133g CTAB, 9.2267g n-butanol, 30ml isooctane into a three-necked flask, add 4.558g ions A salt solution with a concentration of 1.5 mol / L, mix and stir until CTAB is completely dissolved to form an R value of 20 (molar ratio of water phase to s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com