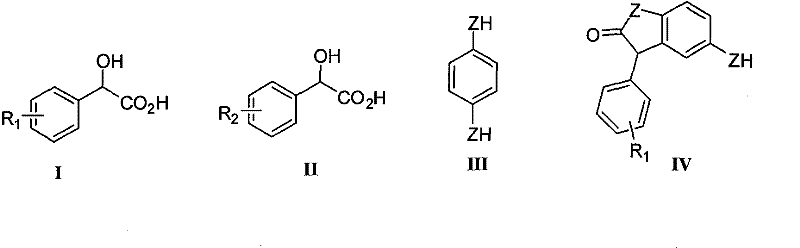
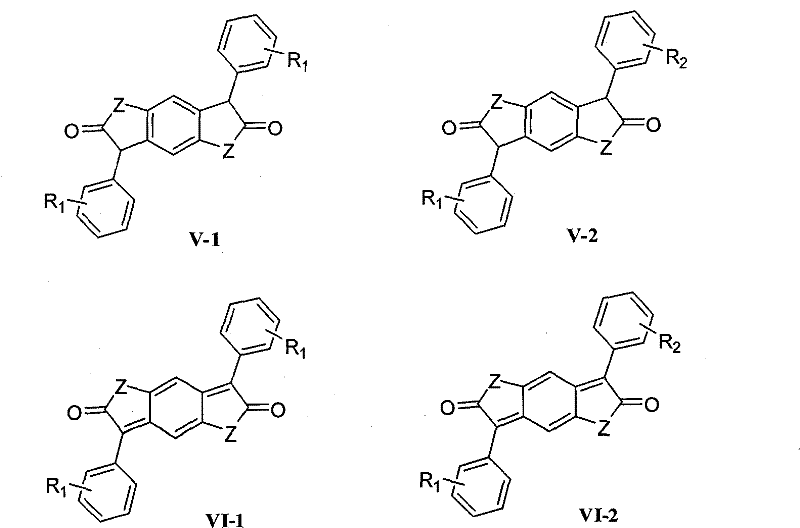
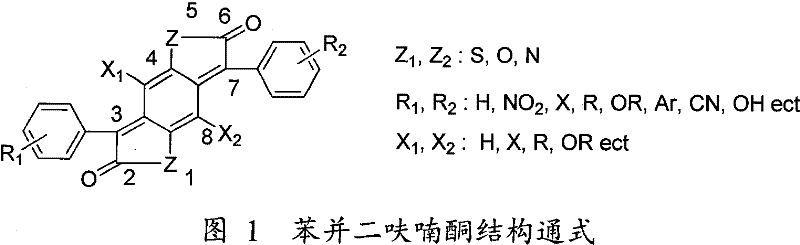
New green synthesizing process for ketocoumaran compound
A technology for the synthesis of benzodifuranone, which is applied in organic chemistry, chemical instruments and methods, azo dyes, etc., can solve the problems of large amount of waste acid, low yield, and not very high yield, and achieve environmental pollution Small size, good perspiration fastness, and the effect of reducing the amount of acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 0.05g 98% concentrated sulfuric acid (0.5mmol), 0.3043g (2mmol) mandelic acid, 0.1101g (1.0mmol) hydroquinone in the mortar successively, grind evenly, react at a constant temperature of 130°C for 4h, wait for the product to cool, use Scrape off with a spatula, transfer to a 10mL test tube, add 2g of acetic acid, 0.23g (2mmol) of 30% aqueous hydrogen peroxide solution, react at 50°C for 5h, cool the reaction solution to below 10°C, filter with suction to obtain a reddish-brown solid and dry it in vacuum to obtain Crude product 0.2376g, crude yield 69.88%. Recrystallized from toluene to obtain a dark red flaky solid.
Embodiment 2
[0033] Add 0.03g polyphosphoric acid (0.3mmol), 0.4553g (2.5mmol) p-methoxymandelic acid, 0.1101g (1.0mmol) hydroquinone in the mortar in turn, grind evenly, react at a constant temperature of 80°C for 8h, and wait for production The substance was cooled, scraped off with a spatula, transferred to a 10mL test tube, added 2g of acetic acid, 0.23g (2mmol) of 30% hydrogen peroxide aqueous solution, and reacted at 50°C for 5h, cooled the reaction solution to below 10°C, and filtered the obtained solid under vacuum After drying, a crude product was obtained, which was recrystallized from toluene to obtain 0.2560 g of a dark red flaky solid.
Embodiment 3
[0035] Add 0.027g zinc chloride (0.2mmol), 0.2103g (1.0mmol) p-propoxymandelic acid, 0.1101g (1.0mmol) hydroquinone in the mortar, grind evenly, and react at a constant temperature of 100°C for 2 hours. Add 0.1863g (1.0mmol) p-chloromandelic acid, grind evenly, and react at a constant temperature of 100°C for 4h. After the product is cooled, scrape it off with a scraper, transfer it to a 10mL test tube, add 2g of acetic acid, 0.23g of 30% aqueous hydrogen peroxide ( 2mmol), reacted at 50°C for 5h, cooled the reaction liquid to below 10°C, and vacuum-dried the solid obtained by suction filtration to obtain a crude product, which was recrystallized with toluene to obtain 0.1854g of a solid product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com