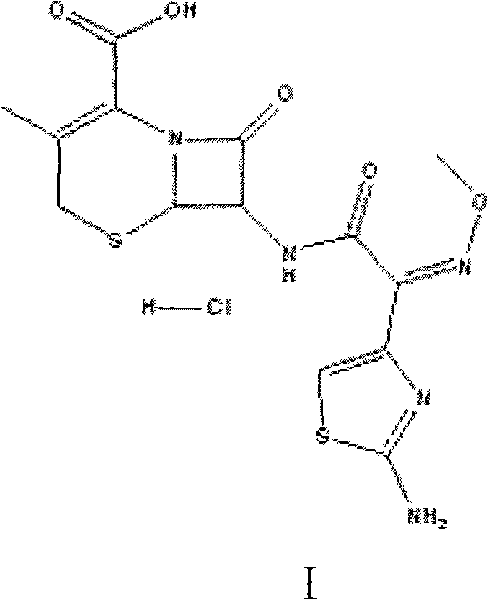
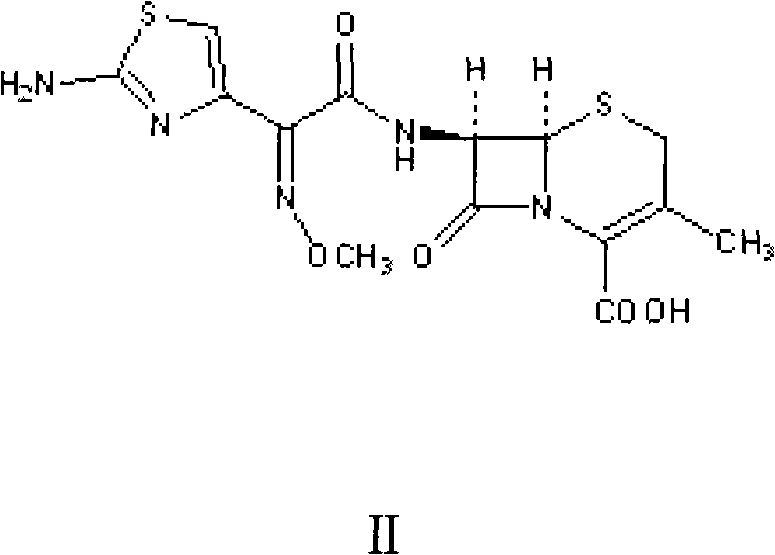
Method for preparing intermediate of cefetamet pivoxil hydrochloride
A technology of ceftamet pivoxil hydrochloride and ceftametexil acid, which is applied in the field of preparation of ceftametexil pivoxil hydrochloride intermediates, can solve the ceftametexil acid synthesis process that has not been reported, increased water and ethanol consumption, and crystallization conditions requirements Harsh and other problems, to achieve the effect of industrial production, avoid energy waste, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
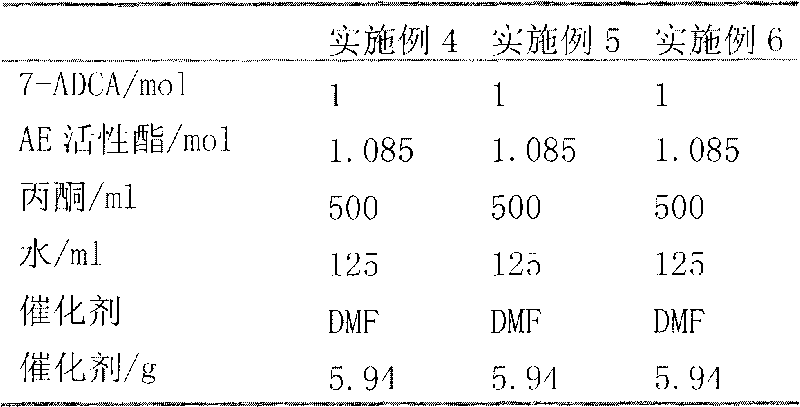
[0052] Take 214.25g (1mol) of 3-methyl-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (7-ADCA) , 500ml of acetone, and 125ml of pure water were added to a 2000ml three-neck flask, fully stirred and cooled to 20°C, slowly added dropwise 101.19g (1mol) of triethylamine, cooled while stirring, cooled to 20°C after fully stirring, and 367.5g (1.05mol) of 2-methoxyimino-2-(2-amino-4-thiazolyl)-(z)-thioxoacetate (AE active ester) was added to the above-mentioned 7- Add 2.9g of N,N-dimethylformamide to the mixed solution of ADCA as a catalyst. After fully stirring, incubate and react at 20°C for 2.5h. After the reaction is completed, extract with 50ml of water and 50ml of dichloromethane. , the organic phase was discarded, and the water phase part was distilled off under reduced pressure to remove acetone and dichloromethane, and the acetone and dichloromethane gases were condensed into liquids by a condenser and then recovered. Then in the aqueous solution that...
Embodiment 2
[0054] Take 214.25g (1mol) of 3-methyl-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (7-ADCA) , 500ml of acetone, and 100ml of pure water were added to a 2000ml three-necked flask, fully stirred and cooled to 20°C, slowly added dropwise 121.43g (1.2mol) of triethylamine, cooled while stirring, and cooled to 20°C after fully stirring, Add 353.5 g (1.01 mol) of 2-methoxyimino-2-(2-amino-4-thiazolyl)-(z)-dihydrazolylthioacetate (AE active ester) to the above-mentioned mixture containing 7 -ADCA mixed solution, and add 8.52g N,N-dimethylbenzamide as a catalyst, after fully stirring, keep warm at 18°C for 1h, extract with 50ml water and 50ml dichloromethane after the reaction, and divide statically phase, the organic phase was discarded, and the water phase part was distilled off under reduced pressure to remove acetone and dichloromethane, and the acetone and dichloromethane gases were condensed into liquids by the condenser and recovered. Then in the aque...
Embodiment 3
[0056] Get 214.25g (1mol) of 3-methyl-7-amino-8-oxo-5-thia-1azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (7-ADCA), Add 300ml of acetone and 300ml of pure water into a 2000ml three-neck flask, stir fully and cool down to 20°C, slowly add 106.25g (1.05mol) of triethylamine dropwise, cool down while stirring, and cool down to 20°C after fully stirring. 381.5g (1.09mol) of 2-methoxyimino-2-(2-amino-4-thiazolyl)-(z)-thiophenhydrazine thioacetate (AE active ester) was added to the above-mentioned 7- Add 6.44g of N,N-dimethylacetamide as a catalyst to the mixed solution of ADCA, and after fully stirring, incubate and react at 22°C for 4h. The organic phase was discarded, and the water phase part was distilled off under reduced pressure to remove acetone and dichloromethane, and the acetone and dichloromethane gases were condensed into liquids by a condenser and then recovered. Then in the aqueous solution that removes acetone and dichloromethane, add medicinal active carbon, the add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com