Medicine composite for treating early liver fibrosis or early hepatocirrhosis decompensation, preparation method and application
A technology of liver fibrosis and composition, applied in the field of pharmaceutical composition for the treatment of liver fibrosis and liver cirrhosis in the early stage of decompensation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 pharmaceutical tablet preparation method of the present invention
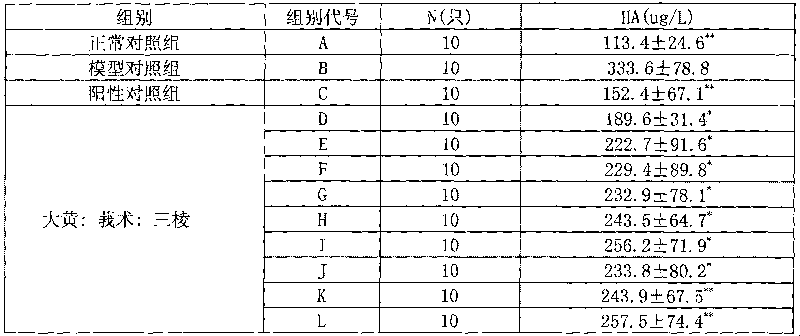
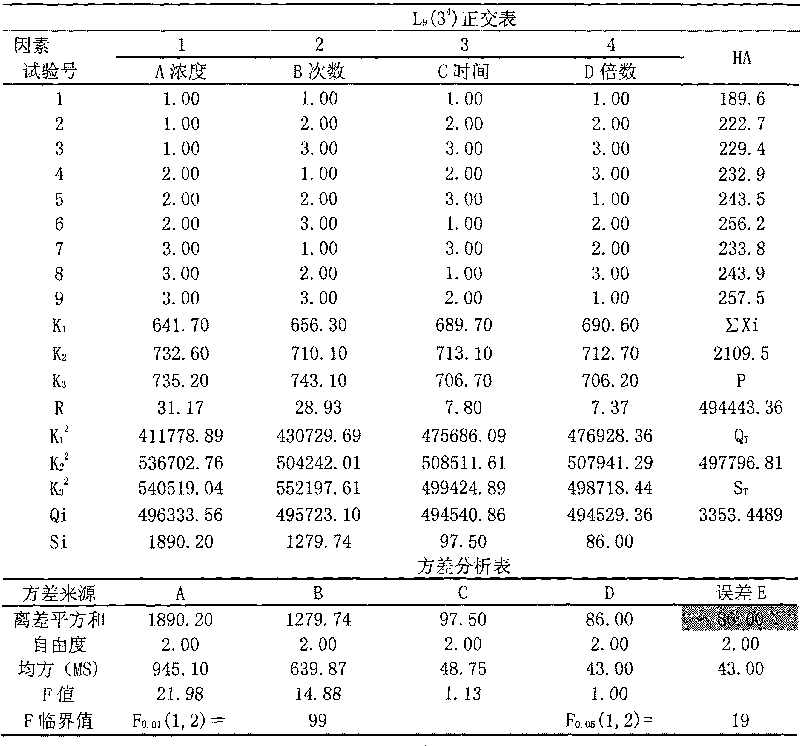
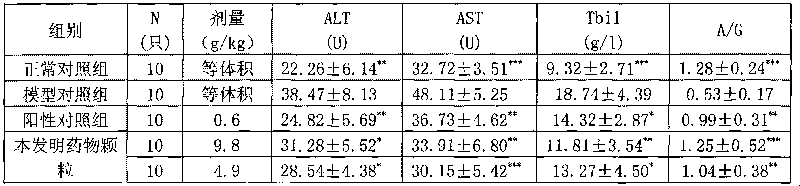
[0025] Prescription: Rhubarb 15g Sanleng 10g Curcuma 10g
[0026] Preparation method 1: Rhubarb herb decoction pieces, use water as the extraction solvent, add water and decoct three times, the first time for 2 hours, the second time for 1 hour, and the third time for 1 hour, combine the decoctions, filter, and concentrate the filtrate to a relative density of 1.38 ~ 1.40 (50 ℃) clear ointment, set aside. Separately take the decoction pieces of Sanleng and Zezhu medicinal materials, use water as the solvent, add 8 times the amount of water, and distill with water, collect the volatile oil, filter the decoction, and concentrate the filtrate to a clear paste with a relative density of 1.38-1.40 (50°C). Combine the above clear pastes, add appropriate amount of auxiliary materials, mix well, vacuum dry, pulverize into fine powder, granulate, dissolve the volatile oil in ethanol, spray into the...
Embodiment 2
[0028] Embodiment 2 pharmaceutical capsule preparation method of the present invention
[0029] Prescription: rhubarb 50g triangular 150g curcuma 150g
[0030] Preparation method 1: Rhubarb herb decoction pieces, use water as the extraction solvent, add water and decoct three times, the first time for 2 hours, the second time for 1 hour, and the third time for 1 hour, combine the decoctions, filter, and concentrate the filtrate to a relative density of 1.38 ~ 1.40 (50 ℃) clear ointment, set aside. Separately take the decoction pieces of Sanleng and Zezhu medicinal materials, use water as the solvent, add 8 times the amount of water, and distill with water, collect the volatile oil, filter the decoction, and concentrate the filtrate to a clear paste with a relative density of 1.38-1.40 (50°C). Combine the above clear pastes, add appropriate amount of auxiliary materials, mix well, vacuum dry, pulverize into fine powder, granulate, dissolve the volatile oil in ethanol, spray in...
Embodiment 3
[0032] Embodiment 3 preparation method of pharmaceutical granule of the present invention
[0033] Prescription: Rhubarb 150g Sanleng 50g Curcuma 50g
[0034] Preparation method 1: Rhubarb herb decoction pieces, use water as the extraction solvent, add water and decoct three times, the first time for 2 hours, the second time for 1 hour, and the third time for 1 hour, combine the decoctions, filter, and concentrate the filtrate to a relative density of 1.38 ~ 1.40 (50 ℃) clear ointment, set aside. Separately take the decoction pieces of Sanleng and Zezhu medicinal materials, use water as the solvent, add 8 times the amount of water, and distill with water, collect the volatile oil, filter the decoction, and concentrate the filtrate to a clear paste with a relative density of 1.38-1.40 (50°C). Combine the above clear pastes, add appropriate amount of auxiliary materials, mix well, vacuum dry, pulverize into fine powder, granulate, dissolve the volatile oil in ethanol, spray int...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com