Compositions and methods for treating ocular diseases and conditions
A composition and eye disease technology, applied in chemical instruments and methods, diseases, metabolic diseases, etc., can solve problems such as affinity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0401] Embodiment 1: against the murine monoclonal antibody of S1P (Sphingomab TM ;LT1002)
[0402] One type of therapeutic antibody specifically binds undesired sphingolipids to achieve superior effects, such as (1) reducing the effective concentration of undesired toxic sphingolipids (and / or the concentration of their metabolite precursors), The sphingolipid promotes undesired effects such as cardiotoxicity, tumorigenicity, or angiogenic effects; (2) inhibits the binding of undesired, toxic, tumorigenic, or angiogenic sphingolipids to cell receptors, thus , and / or reduce the concentration of sphingolipids available for binding to such receptors. Examples of such therapeutic effects include, but are not limited to, the use of anti-S1P antibodies to reduce the effective in vivo serum concentration of available S1P, thereby blocking or at least limiting the tumorigenic and angiogenic effects of S1P, and its role in post-MI heart failure, cancer, or a role in fibrogenesi...
Embodiment 2
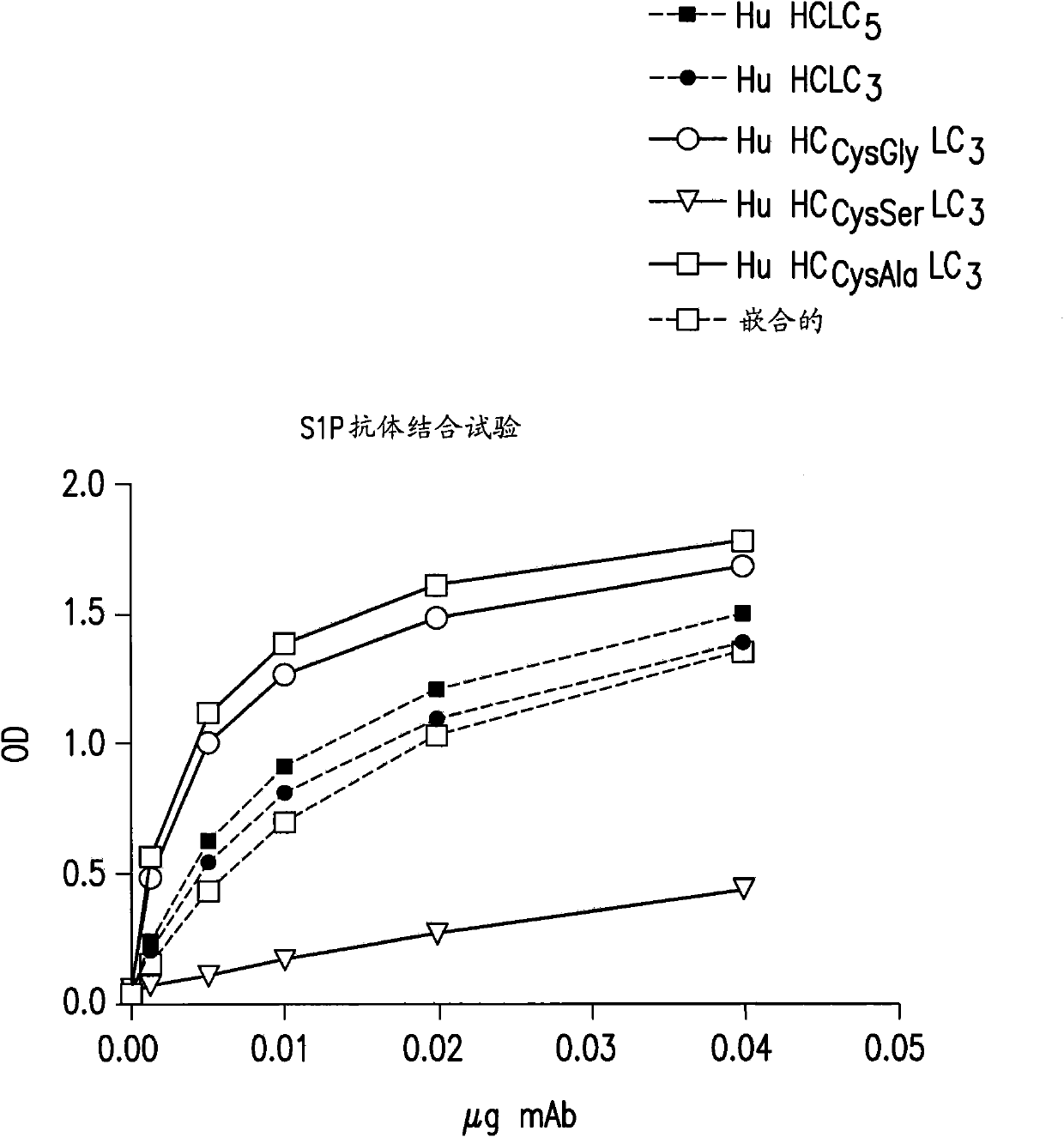
[0412] Example 2: ELISA assay
[0413] 1. Quantitative ELISA
[0414] Microtiter ELISA plates (Costar, Cat No. 3361) were coated with rabbit anti-mouse IgG and F(ab') was diluted in 1M carbonate buffer (pH 9.5) at 37°C 2 Fragment-specific antibody (Jackson, 315-005-047) 1 h. Plates were washed with PBS and blocked with PBS / BSA / Tween-20 for 1 h at 37°C. For the initial incubation, non-specific mouse IgG or human IgG, complete molecules (for calibration curves) and dilutions of the samples to be tested are added to the wells. Plates were washed and incubated with 100 μl per well of HRP-conjugated goat anti-mouse (H+L) (1 :40000 dilution) at 37°C (Jackson, cat No 115-035-146) for 1 h. After washing, the enzymatic reaction was detected using tetramethylbenzidine (Sigma, cat No T0440) and detected by adding 1M H 2 SO 4 termination. Optical density (OD) was measured at 450nm using a ThermoMultiskan EX. Raw data were converted into GraphPad software for analysis.
[0415]...
Embodiment 3
[0419] Example 3: SPHINGOMAB murine mAb is highly specific for S1P
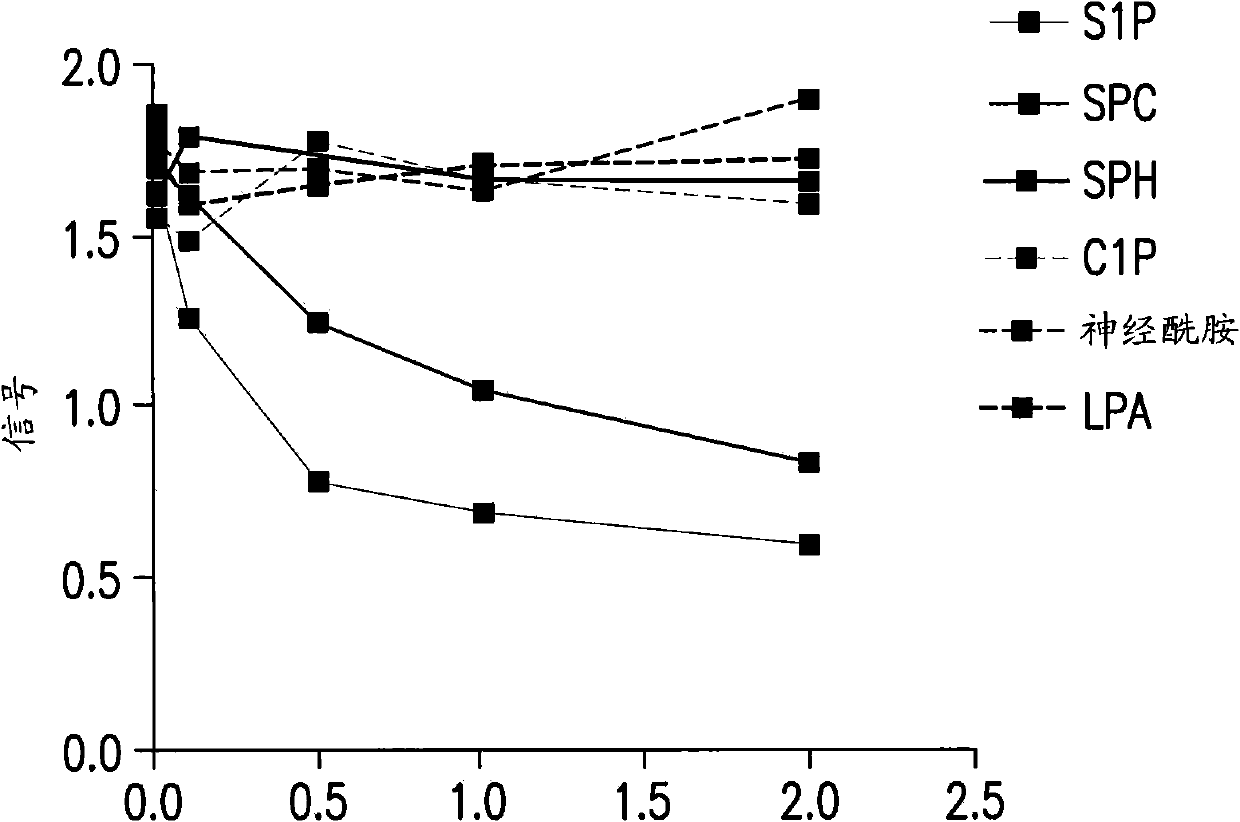
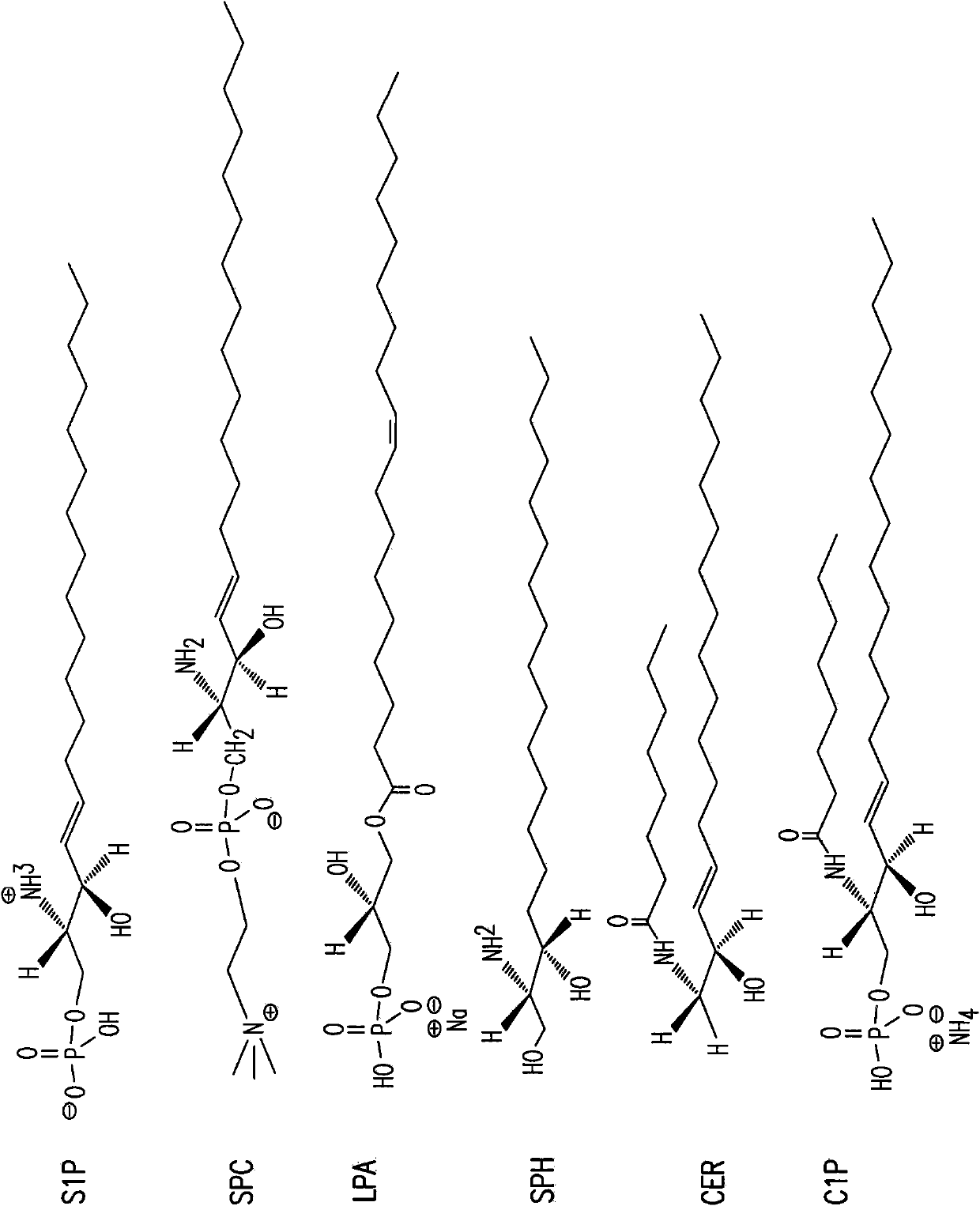
[0420] A competitive ELISA confirmed the specificity of SPHINGOMAB for S1P compared to other bioactive lipids. SPHINGOMAB demonstrated no cross-reactivity to sphingosine (SPH), the immediate metabolic precursor of S1P or lysophosphatidic acid (LPA), an important extracellular signaling molecule similar in structure and function to S1P. SPHINGOMAB does not recognize other structurally similar lipids and metabolites, including ceramide-1-phosphate (C1P), dihydrosphingosine (DH-SPH), phosphatidylserine (PS), phosphatidylethanolamine (PE) or Sphingomyelin (SM). SPHINGOMAB does cross-react with dihydrosphingosine-1-phosphate (DH-S1P) and to a lesser extent with sphingosine phosphorylcholine (SPC) ( image 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com