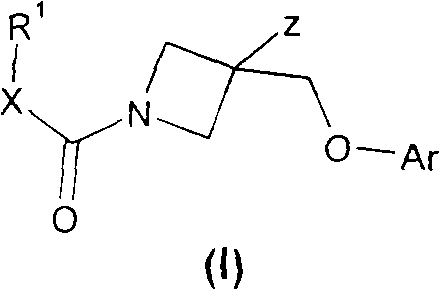
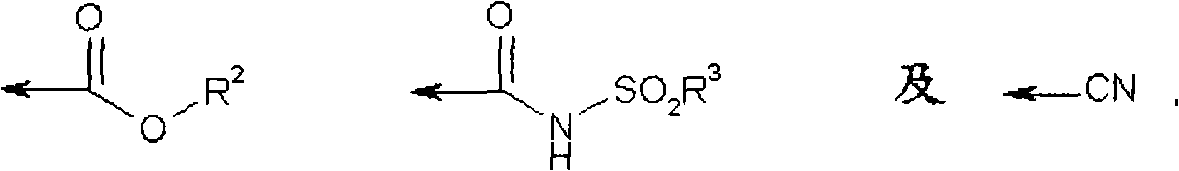
Azetidine derivatives and their use as prostaglandin E2 antagonists
A prodrug, alkyl technology, applied in the direction of anti-inflammatory agents, medical preparations containing active ingredients, anti-tumor drugs, etc., can solve the problems of efficacy and selective discomfort medical treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0384] Example 1: 1-(4-chlorobenzoyl)-3-{[(4′-cyanobiphenyl-4-yl)oxy]methyl}azetidine-3-carboxylic acid
[0385]
[0386] 4'-Hydroxy-4-biphenylcarbonitrile (30.9 mg, 0.15 mmol) and potassium carbonate (109 mg, 0.79 mmol) were added to 1-(4- Into a stirred solution of ethyl chlorobenzoyl)-3-(chloromethyl)azetidine-3-carboxylate (50 mg, 0.15 mmol) (see Preparation 8). The resulting mixture was stirred at 130°C for 0.25 hours. Then, water (1 mL) was added and the resulting mixture was heated at 80 °C for 10 min. It was then allowed to cool before partitioning between aqueous hydrochloric acid (2M, 5 mL) and dichloromethane (5 mL). The organic layer was dried over sodium sulfate, and concentrated under reduced pressure. The residual brown oil was purified by HPLC (method a).
[0387] LCMS Rt 3.54 min, ES m / z 446 [M+H] +
[0388] Examples 2 to 13 were prepared according to the method described above for Example 1 from the appropriate halogenated compound of formula (II) an...
Embodiment 14a
[0397] Example 14a: 1-(4-Fluorobenzoyl)-3-{[(6-methoxy-2-naphthyl)oxy]methyl}azetidine-3-carboxylic acid
[0398]
[0399] 2-Hydroxy-6-methoxynaphthalene (32.3 g, 0.19 mol) and potassium carbonate (51.3 g, 0.37 mol) were added to 1-(4-fluorobenzoyl )-3-(Chloromethyl)azetidine-3-carboxylic acid ethyl ester (37.1 g, 0.12 mol) (see Preparation 5). The resulting mixture was stirred at 120°C for 50 minutes. Water (50 mL) was added and the reaction mixture was heated at 80 °C for 1 h, then allowed to cool before partitioning between aqueous hydrochloric acid (2M, 1.2 L) and ethyl acetate (1.5 L). The organic layer was dried over sodium sulfate, and concentrated under reduced pressure. The residual brown solid was triturated with diethyl ether (1.5 L) and the resulting pink solid was collected by filtration. The pink solid was then purified by silica gel column chromatography eluting with 97.5 / 2.5 / 0.25 dichloromethane / methanol / acetic acid to provide the title compound as a pale...
Embodiment 14b
[0401] Example 14b: 1-(4-Fluorobenzoyl)-3-{[(6-methoxy-2-naphthyl)oxy]methyl}azetidine-3-carboxylic acid
[0402]
[0403] Ethyl 1-(4-fluorobenzoyl)-3-{[(6-methoxy-2-naphthyl)oxy]methyl}azetidine-3-carboxylate (50.0 g, 114 mg mol) (see Preparation 33) was suspended in acetonitrile (500 mL), and sodium trimethylsilanolate (14.1 g, 126 mmol) and water (2.05 mL, 114 mmol) were added. The suspension was stirred at ambient temperature for 4 hours. Then, 10% (v / v) aqueous phosphoric acid solution (100 mL, 171 mmol) was added, and the reaction mixture was stirred at ambient temperature for 1 hour, then at 0 °C for an additional 2 hours. The precipitate was collected and washed twice with water (2x250 mL) and dried under reduced pressure to provide the title compound as a white solid in 85% yield, 39.5 g.
[0404] 1 H NMR (400MHz, CD 3 OD)δ: 3.84(s, 3H), 4.27(d, 1H), 4.42(m, 2H), 4.44(s, 2H), 4.67(d, 1H), 7.07(m, 2H), 7.20(m, 4H), 7.63(m, 2H), 7.72(m, 2H); ES m / z 410[M+H] + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com