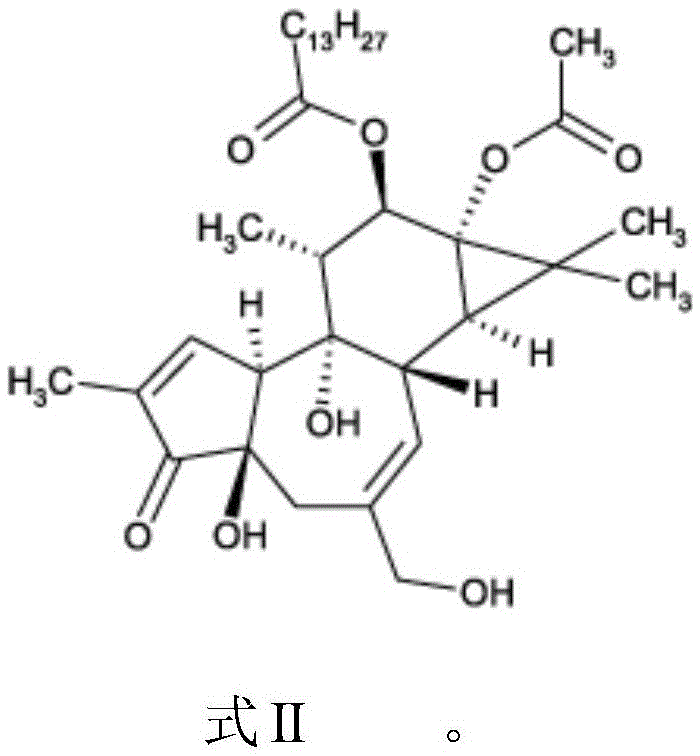
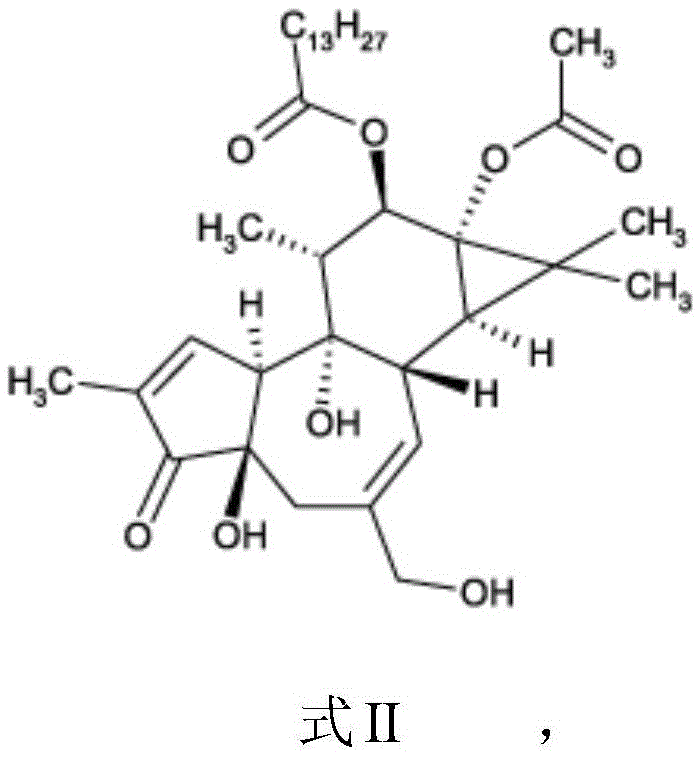
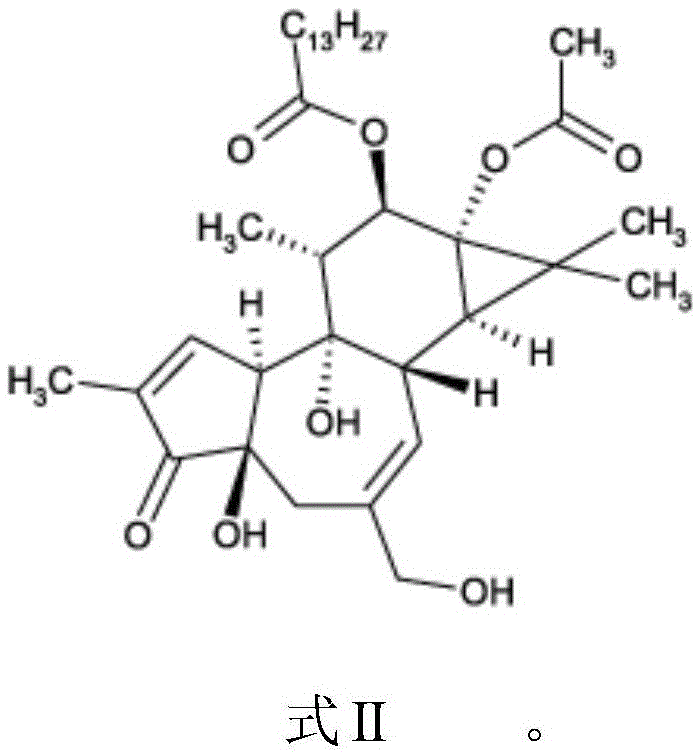
Compounds of phorbol esters and methods of use
A technology of phorbol ester and composition, applied in the field of cell pathology, can solve severe nausea, decreased bone marrow function, toxicity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Effects of TPA on the number of peripheral white blood cells (WBC) and hemoglobin (Hb) in mice injected with S180 cells:
[0107] Sarcoma 180 (S180) cells were injected into Kunming (Kwen-Ming) mice. On day three, mice were given 50, 100 or 200 μg / kg / day of TPA intraperitoneally (i.p.) for 7 days. The day after completion of treatment, blood samples were taken from the tails of treated mice for WBC and Hb analysis. The WBC counts in the treatment groups (50, 100, or 200 μg / kg / day for 7 days) were 16.1±7.4, 18.7±3.0, and 20.7±3.4x10 per liter, respectively. 9 ; while the WBC count in the control group was 13.6±1.8x10 per liter 9 . The Hb in the treatment group was 136±11, 149±12 and 149±10 grams per liter, while the Hb in the control group was 134±15 grams / liter. The results indicate that the i.p. injection of TPA can increase the number of peripheral white blood cells (WBC) in the mice according to the degree of dose. However, after comparing with the mice in the co...
Embodiment 2
[0110] Due to the strong local irritation caused by TPA, TPA is administered to patients by intravenous (i.v.) injection. The TPA solution in the sterile syringe was injected into 200 ml of sterile normal saline and mixed well for i.v. injection.
[0111] Clinical toxicity and side effects of different TPA dosages:
[0112] (1) Dosing at a dose of 1 mg of TPA per patient per week:
[0113] One milligram of TPA in solvent was mixed well with 200 milliliters of sterile saline for intravenous injection at a rate of 16 micrograms per minute over 1 hour. One hour after TPA administration, the patient began to have chills lasting 30 minutes, followed by fever (the patient's body temperature reached 37.5-39.5°C and lasted for 3-5 hours, and then returned to normal), accompanied by varying degrees of sweating . The above symptoms can be alleviated by giving patients glucocorticoid. TPA at this dose caused a small number of patients to bleed, while se...
Embodiment 3
[0119] Phase 1 clinical trial of HIV+patients treated with TPA
[0120] Twelve symptomatic patients (five men and seven women) were treated with TPA, aged 35 to 52, all of whom were HIV-infected by blood transfusion in 1995, And are resistant to standard HIV treatment. Each patient was administered intravenously (i.v.) a weight-adjusted dose of TPA (75 μg / sqm) in 200 mL of sterile saline over 1 hour. This dose is given once daily for the first to three days of treatment. Each patient was then given this dose every two days from day 4 to day 8, followed by a 6-month rest period before the second phase of treatment according to the same program.
[0121] Blood samples were collected prior to the first dose and on Days 4 and 40 of the treatment cycle. The amount of CD3, CD4 and CD8 in peripheral blood was measured by monoclonal antibody (Becton Dickson Scientific Co., Franklin Lakes, NJ) and flow cytometer (B.D. Bioscience, San Diego, CA).
[0122] As shown in Table 1, no con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com