Triphenylamine derivative, a method for manufacturing triphenylamine derivates and electronic photographic photoreceptor
A manufacturing method and electrophotography technology, applied in the direction of chemical instruments and methods, preparation of organic compounds, preparation of amino hydroxyl compounds, etc., can solve the problem of crystallization of photosensitive layer, poor dispersion, insufficient compatibility of solvent-soluble binder resin, It is difficult to obtain the sensitivity characteristics and other issues, to achieve excellent sensitivity characteristics, improve charge transport efficiency, and improve the effect of charge movement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0058] The first embodiment is a triphenylamine derivative represented by the following general formula (1).
[0059]
[0060] (In general formula (1), R 1 ~R 13 independently hydrogen atom, alkyl group with 1 to 8 carbon atoms, alkoxy group with 1 to 8 carbon atoms, phenoxy group, aryl group with 6 to 20 carbon atoms or aryl group with 7 to 20 carbon atoms Alkyl group, Ar may have an alkyl group with 1 to 8 carbon atoms, an alkoxy group with 1 to 8 carbon atoms, a hydrocarbon ring with 3 to 14 carbon atoms, or a heterocyclic ring with 3 to 10 membered rings as a substituent In the aryl group having 6 to 20 carbon atoms, the number of substituents n is an integer of 0 to 4, and the repetition numbers o and p are independently integers of 0 to 1. )
[0061] Hereinafter, the triphenylamine derivative which has the specific structure of 1st Embodiment is demonstrated concretely.
[0062] The triphenylamine derivative of the first embodiment is characterized in that it is a...
no. 2 approach
[0106] The second embodiment is a method for producing triphenylamine derivatives, which is characterized in that it is a method for producing triphenylamine derivatives represented by the general formula (1) of the first embodiment, including performing the process represented by the following reaction formula (1) The process of reaction.
[0107]
[0108] (In general formula (2) and (5), X 1 and x 2 They are independently independent halogen atoms, and the other substituents in the general formulas (2) to (5) are the same as those described in the general formula (1). )
[0109] Hereinafter, the second embodiment will be specifically described focusing on differences from those described in the first embodiment.
[0110] 1. Preparation process
[0111] This preparatory step is a step for obtaining compounds represented by the following general formulas (2) and (5) as material substances in the reaction formula (1).
[0112] In addition, since the compounds represente...
no. 3 approach
[0165] A third embodiment is an electrophotographic photoreceptor having a photosensitive layer on a substrate, wherein the photosensitive layer contains a triphenylamine derivative represented by the general formula (1).
[0166] Hereinafter, the third embodiment will be specifically described by dividing it into a single-layer electrophotographic photoreceptor and a laminated electrophotographic photoreceptor, focusing on the differences from those described in the first embodiment and the second embodiment.
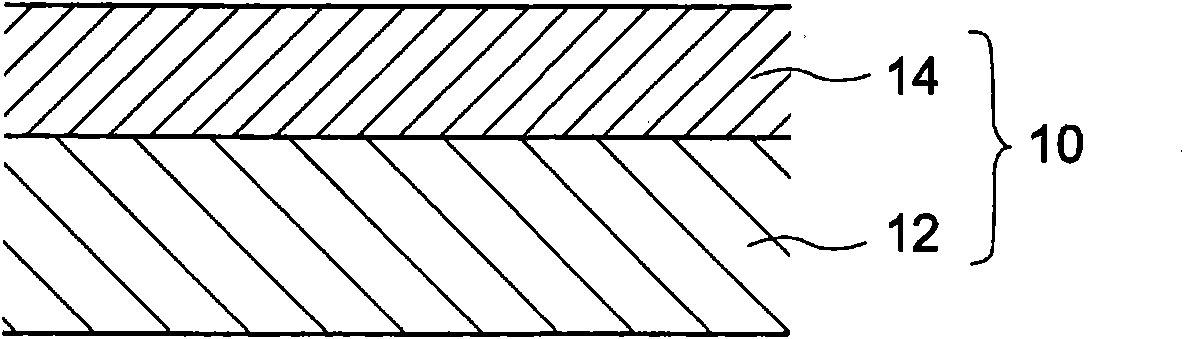
[0167] 1. Single-layer electrophotographic photoreceptor
[0168] The electrophotographic photoreceptor containing the triphenylamine derivative according to the first to second embodiments is preferably constituted as a single-layer electrophotographic photoreceptor.
[0169] The reason is that, by constituting the electrophotographic photoreceptor containing the triphenylamine derivatives according to the first to second embodiments as a single-layer electrophotographi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com