Method for preparing 2,2-dimethyl-3-hydrocinnnamic aldehyde and derivative thereof
A technology of phenylpropanal and derivatives, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of high price of methyl iodide, unfavorable environmental protection, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of 2,2-Dimethyl-3-(2,4-Dimethylphenyl)-1-propanal (Compound 1)
[0031] The first step: the synthesis of 1-(bromomethyl)-2,4-dimethylbenzene
[0032] Add 50 mL of glacial acetic acid, 10.6 g (0.1 mol) of m-xylene, and 3.1 g (0.1 mol) of paraformaldehyde into a 100 mL one-necked flask. After rapid stirring, 16 mL of 40% HBr solution was quickly added to the mixed solution, and the mixture was kept at 80° C. for 8 hours. Then pour it into 100mL of water, separate the liquids, dry the organic phase with anhydrous magnesium sulfate, filter, and decompress the filtrate to obtain 12.3g of 1-(bromomethyl)-2,4-dimethylbenzene with a yield of 62%.
[0033] The second step: the synthesis of 2,2-dimethyl-3-(2,4-dimethylphenyl)-1-propanal
[0034] Under the condition of nitrogen protection, add ground powdery NaOH 2g (50mmol) in the 100mL round bottom flask, phase transfer catalyst tetrabutylammonium bromide (TBAB) 0.5g (10% w / w), toluene 40mL, vigorously stir, The tem...
Embodiment 2
[0037] Synthesis of 2,2-Dimethyl-3-(2,5-Dimethylphenyl)-1-propanal (Compound 2)
[0038] The first step: the synthesis of 1-(bromomethyl)-2,5-dimethylbenzene
[0039] Add 50 mL of glacial acetic acid, 10.6 g (0.1 mol) of p-xylene, and 3.1 g (0.1 mol) of paraformaldehyde into a 100 mL single-necked flask. After rapid stirring, 16 mL of 40% HBr solution was quickly added to the mixed solution, and the mixture was kept at 80° C. for 8 hours. Then it was poured into 100 mL of water, separated, the organic phase was dried with anhydrous magnesium sulfate, filtered, and the filtrate was decompressed to obtain 14.7 g of 1-(bromomethyl)-2,5-dimethylbenzene with a yield of 74%.
[0040] The second step: the synthesis of 2,2-dimethyl-3-(2,5-dimethylphenyl)-1-propanal
[0041] Under the condition of nitrogen protection, 2g (50mmol) of ground powdered NaOH, 0.5g (10% w / w) of phase transfer catalyst tetrabutylammonium bromide (TBAB), 40mL of toluene, and strong Stir and heat up to 70°C....
Embodiment 3
[0044]Synthesis of 2,2-Dimethyl-3-(3,4-Dimethylphenyl)-1-propanal (Compound 3)
[0045] The operation is the same as example 1
[0046] Using o-xylene as the starting substrate, bromination yield: 67% Alkylation yield: 58%, 2,2-dimethyl-3-(2,3-dimethylphenyl)-1- Propionaldehyde: 2,2-dimethyl-3-(3,4-dimethylphenyl)-1-propanal = 4:1. Fragrance description: Translucent floral fragrance, lily of the valley flower fragrance, rabbit ear oxalin.
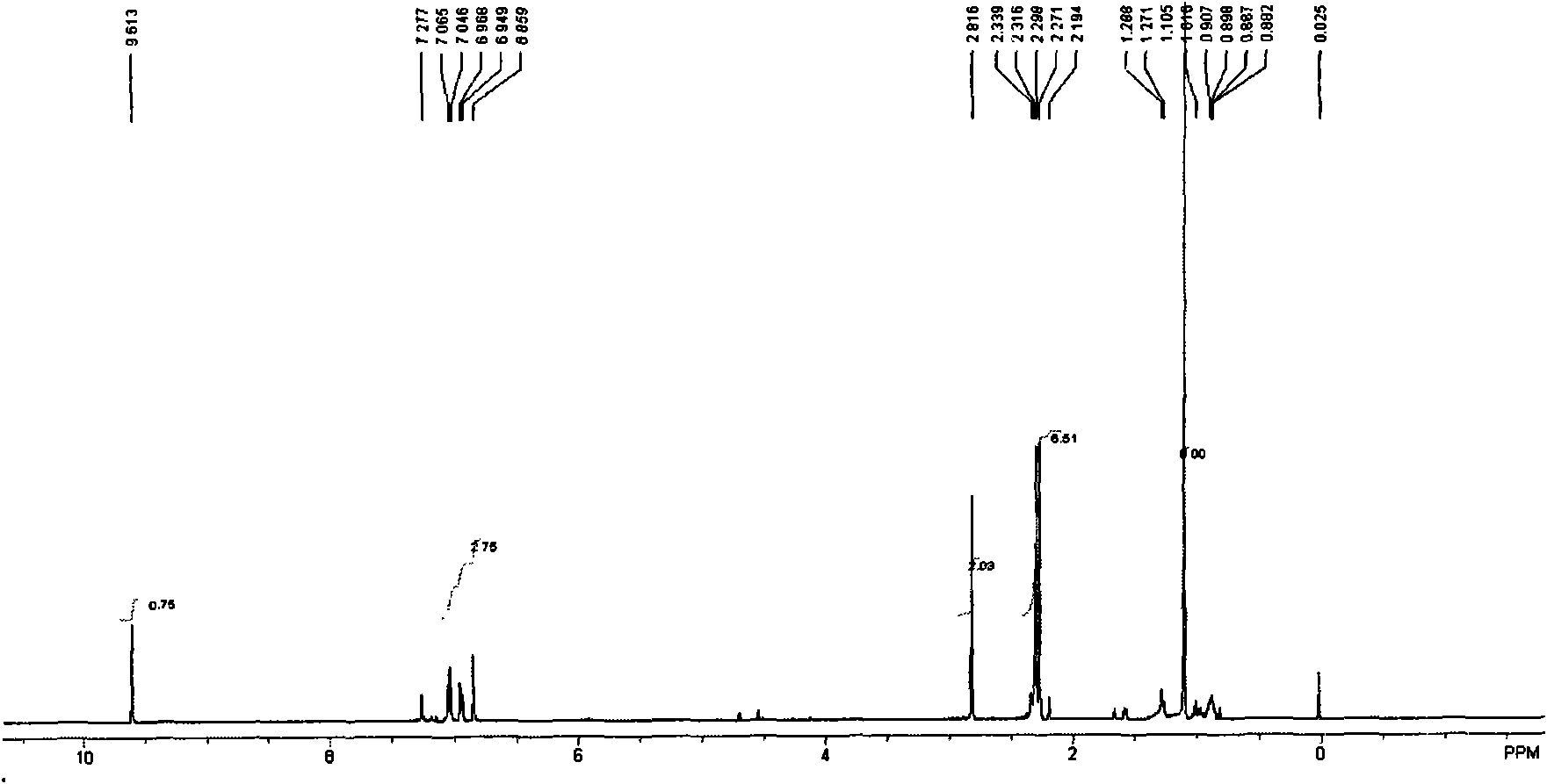
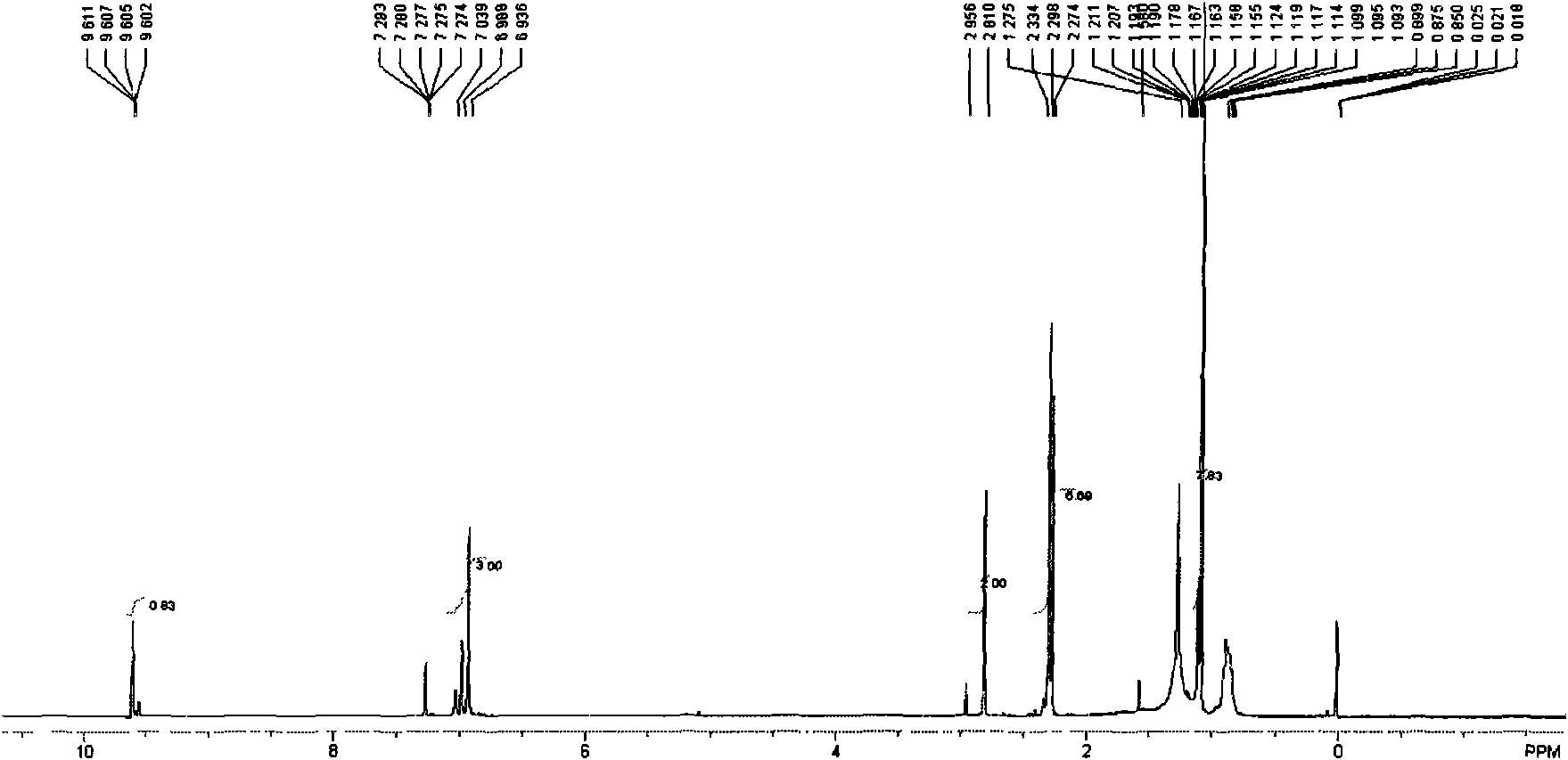
[0047] 1 HNMR (δ, CDCl 3 ): 1.07(s, 6H, 2C(CH 3 )), 2.50(d, 6H, Ar-CH 3 ), 2.73 (s, 2H, Ar-CH2), 6.83-7.07 (m, 3H, Ar-H), 9.61 (s, 1H, CH=O).IR: KBr, v / cm-13040 (Ar-H stretching vibration), 2965, 2925, 2872 (CH 3 , CH 2 stretching vibration), 1726 (C=O stretching vibration), 1610, 1496, 1470 (CH 3 Asymmetric scissor vibration), 1386 (CH 3 Scissor vibration), 1156; (attached image 3 , attached Figure 7 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com