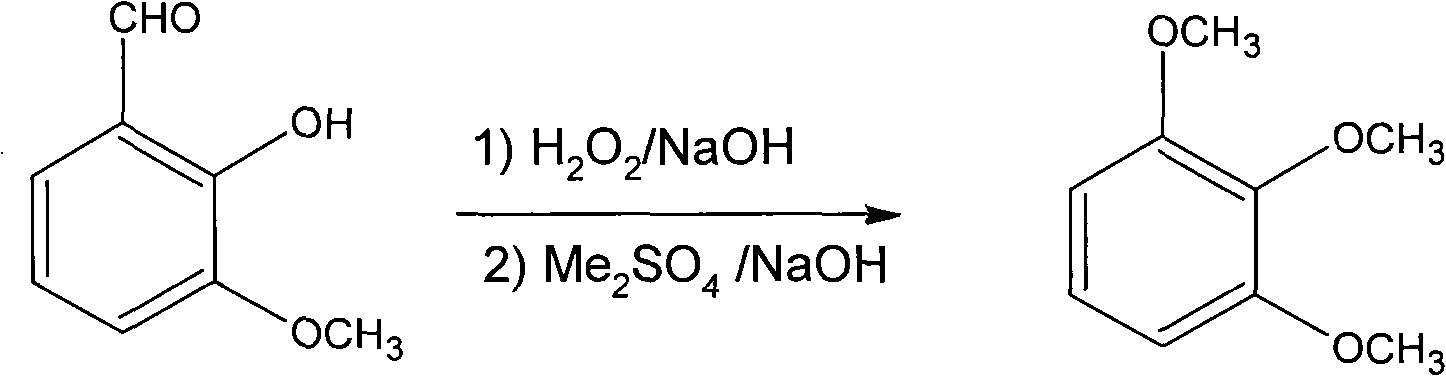One-pot process for synthesizing 1,2,3-trimethoxy-benzene by using o-vanillin
A technology of trimethoxybenzene and o-vanillin, applied in 1 field, achieves the effects of simple operation, low equipment requirements, and simple process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] In actual production, 200 kilograms of o-vanillin, 52 kilograms of sodium hydroxide, and 500 kilograms of water were put into the reaction kettle in turn for stirring. After stirring and mixing evenly, 2200 kilograms of 3% hydrogen peroxide were added dropwise. As the dropwise addition proceeded, the reaction was slightly exothermic, and the color of the reaction solution gradually became darker. After the dropwise addition, keep warm until the reaction is complete. The end point of the reaction was judged by thin-layer chromatography (TLC), and the developer was 4 / 6 ethyl acetate / petroleum ether, and the reaction was considered complete until the raw material point completely disappeared. After the reaction was completed, 415 kilograms of dimethyl sulfate and 440 kilograms of 30% sodium hydroxide aqueous solution were added dropwise, and the rate of addition of the two was controlled so that the pH in the still was maintained at 9~10, and the temperature was controlled...
Embodiment 2
[0021] In actual production, 200 kilograms of o-vanillin, 67 kilograms of sodium hydroxide, and 533 kilograms of water were put into the reaction kettle successively for stirring. After stirring and mixing evenly, 2340 kilograms of 3% hydrogen peroxide were added dropwise. As the dropwise addition proceeded, the reaction was slightly exothermic, and the color of the reaction solution gradually became darker. After the dropwise addition, keep warm until the reaction is complete. The end point of the reaction was judged by thin-layer chromatography (TLC), and the developer was 4 / 6 ethyl acetate / petroleum ether, and the reaction was considered complete until the raw material point completely disappeared. After the reaction was completed, 332 kilograms of dimethyl sulfate and 350 kilograms of 30% sodium hydroxide aqueous solution were added dropwise, and the rate of addition of the two was controlled so that the pH in the still was maintained at 9~10, and the temperature was contr...
Embodiment 3
[0023] In actual production, 200 kg of ortho-vanillin, 40 kg of sodium hydroxide, and 400 kg of water were put into the reaction kettle in turn for stirring. After stirring and mixing evenly, 1800 kg of 3% hydrogen peroxide was added dropwise. As the dropwise addition proceeded, the reaction was slightly exothermic, and the color of the reaction solution gradually became darker. After the dropwise addition, keep warm until the reaction is complete. The end point of the reaction was judged by thin-layer chromatography (TLC), and the developer was 4 / 6 ethyl acetate / petroleum ether, and the reaction was considered complete until the raw material point completely disappeared. After the reaction was completed, 663 kilograms of dimethyl sulfate and 700 kilograms of 30% sodium hydroxide aqueous solution were added dropwise, and the rate of addition of the two was controlled so that the pH in the still was maintained at 9~10, and the temperature was controlled between 35~40°C. After ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com