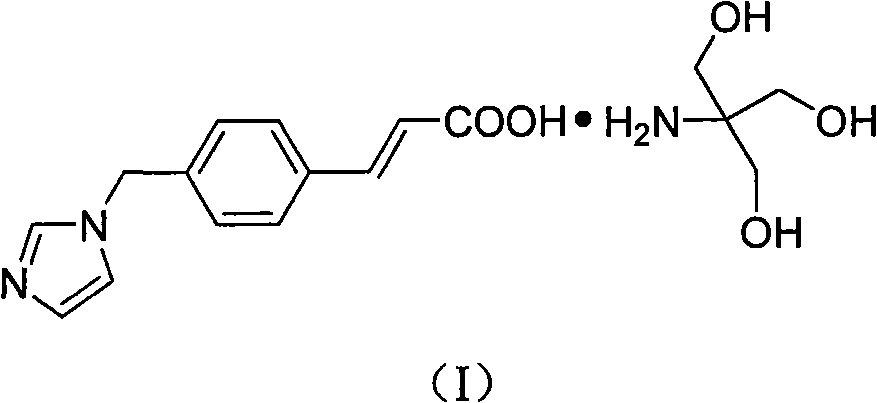
Ozagrel tromethamine, compound, preparation method and application thereof
A technology of tromethamine and tromethamine salt, which is applied in the field of ozagrel tromethamine and compositions thereof, can solve the problems of water-soluble ozagrel salt purity defects, decreased stability and the like, and achieves The synthesis process is simple and feasible, with high stability and high anti-platelet aggregation effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of ozagrel tromethamine
[0042]Dissolve 250.7 g (2.072 mol) of tromethamine in 1125 ml of distilled water, add 450 g (1.974 mol) of ozagrel under stirring at a temperature of 45° C., and keep the reaction for 2 hours after the addition is complete. After cooling to room temperature, a large number of crystals were precipitated, and the crude product was obtained by filtration. Add the crude product to 1125ml of absolute ethanol, heat to reflux for 3 hours, stop the reaction, stir at a temperature of 5°C for 6 hours, filter with suction, rinse with absolute ethanol, and dry to obtain 598g of white powder with a yield of 86.8%. 99.8%, mp: 175-176°C (decomposes on melting). Elemental analysis measured value % (theoretical value %): C58.41 (58.44), H 6.65 (6.64), N12.02 (12.03%). 1 H-NMR (600MHz, DMSO-d 6 )δ: 3.41 (6H, s, 3×CH 2 ), 5.17 (2H, s, CH 2 ), 6.39~6.42 (1H, d, -CH=CHCOOH), 6.89 (1H, s, -N=CH-N), 7.18 (1H, s, N-CH=CH-N-CH 2 ), 7.22~7.23 (2H, d, p...
Embodiment 2
[0044] Preparation of ozagrel tromethamine
[0045] Dissolve 145.2 g (1.2 mol) of tromethamine in 570 ml of distilled water, add 228 g (1 mol) of ozagrel under stirring at a temperature of 35° C., complete the addition, and keep the reaction for 2 hours. Cooling and crystallization, suction filtration to obtain the crude product. Add the crude product to 600ml of absolute ethanol, heat to reflux for 3 hours, stir for 6 hours at a temperature of 10°C, filter with suction, wash with a small amount of absolute ethanol, and dry to obtain 305.3g of white powder with a yield of 87.5% and a content of 99.6% .
Embodiment 3
[0047] Preparation of ozagrel tromethamine
[0048] Dissolve 12.7 g (0.105 mol) of tromethamine in 32 ml of distilled water, add 22.8 g (0.1 mol) of ozagrel under stirring at a temperature of 40° C., complete the addition, and keep the reaction for 2 hours. Cooling and crystallization, suction filtration to obtain the crude product. Add the crude product to 80ml of acetone, stir at 50-60°C for 5 hours, cool, continue to crystallize below 10°C, filter with suction, wash the filter cake with a small amount of acetone, and dry to obtain 30.3g of white powder with a yield of 86.9% and a content of 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com