Method for preparing oxygen carrier freeze-dried preparation of haemoglobin
A technology for hemoglobin and freeze-dried preparations, which can be used in freeze-dried delivery, peptide/protein components, blood diseases, etc., and can solve problems such as difficult storage and poor stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
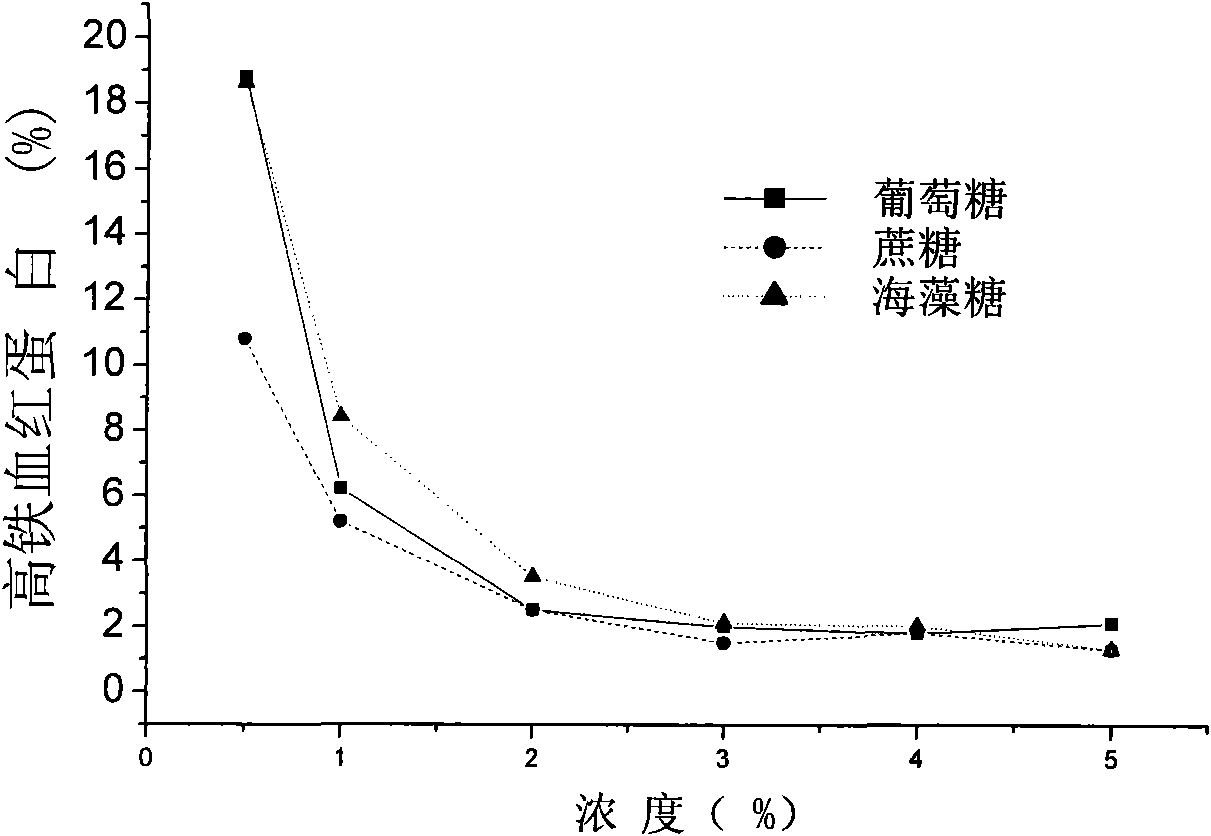
[0101] Hemoglobin oxygen carrier was provided by Xi'an North America Pharmaceutical Co., Ltd. The hemoglobin oxygen carrier was diluted with 20% glucose solution to make the final concentration of 40 mg / ml, and the final concentration of glucose was 4%.
[0102] (1) Subpackage of hemoglobin oxygen carrier: subpackage the prepared product into 5ml centrifuge tubes, 2.5ml per tube;
[0103] (2) Freeze dryer pre-cooling: the vacuum freeze dryer is operated according to the freeze-drying routine, so that the temperature of the cold trap is reduced to about -80°C;
[0104] (3) Pre-freezing: put the product in a -40°C refrigerator for 2 hours;
[0105] (4) Sublimation and drying: turn on the vacuum pump to evacuate. The sublimation drying process is divided into 2 stages:
[0106] Initial drying: shelf temperature -10°C, last 18h;
[0107] Secondary drying: shelf temperature 20°C, last 2h. The vacuum degree of the freeze-drying process is lower than 1Pa;
[0108] (5) After the...
example 2
[0117] Hemoglobin oxygen carrier was provided by Xi'an North America Pharmaceutical Co., Ltd. The hemoglobin oxygen carrier was diluted with 1 mol / LLys-Asp, the concentration of the hemoglobin oxygen carrier was adjusted to 40 mg / ml, and the final concentration of the amino acid salt Lys-Asp was 0.2 mol / L.
[0118] (1) Packing of hemoglobin-like oxygen carriers: Pack the prepared product into 5ml centrifuge tubes, each containing 2.5ml.
[0119] (2) Freeze dryer pre-cooling: the vacuum freeze dryer is operated according to the freeze-drying routine, so that the temperature of the cold trap is reduced to about -80°C;
[0120] (3) Pre-freezing: put the product in a -40°C refrigerator for 2 hours.
[0121] (4) Sublimation and drying: turn on the vacuum pump to evacuate. The sublimation drying process is divided into two stages, primary drying: shelf temperature -10°C, lasts 18h; secondary drying: shelf temperature 20°C, lasts 2h. The vacuum degree of the freeze-drying process ...
example 3
[0131] Hemoglobin oxygen carrier was provided by Xi'an North America Pharmaceutical Co., Ltd. The hemoglobin oxygen carrier was diluted with 20% trehalose solution, the concentration of the hemoglobin oxygen carrier was adjusted to 40 mg / ml, and the final concentration of trehalose was 4%.
[0132] (1) Packing of hemoglobin-like oxygen carriers: Pack the prepared product into 5ml centrifuge tubes, each containing 2.5ml.
[0133] (2) Freeze dryer pre-cooling: the vacuum freeze dryer is operated according to the freeze-drying routine, so that the temperature of the cold trap is reduced to about -80°C;
[0134] (3) Pre-freezing: put the product in a -40°C refrigerator for 2 hours.
[0135] (4) Sublimation and drying: turn on the vacuum pump to evacuate. The sublimation drying process is divided into two stages, primary drying: shelf temperature -10°C, lasts 18h; secondary drying: shelf temperature 20°C, lasts 2h. The vacuum degree of the freeze-drying process is lower than 1Pa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Final concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com