Capsule containing candesartan cilexetil and preparation method thereof
A technology of candesartan cilexetil and capsules, which is applied in the field of capsules containing candesartan cilexetil and its preparation, can solve the problems of gelatin capsules, such as changes in the in vitro dissolution behavior, and achieve the effect of stable dissolution performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
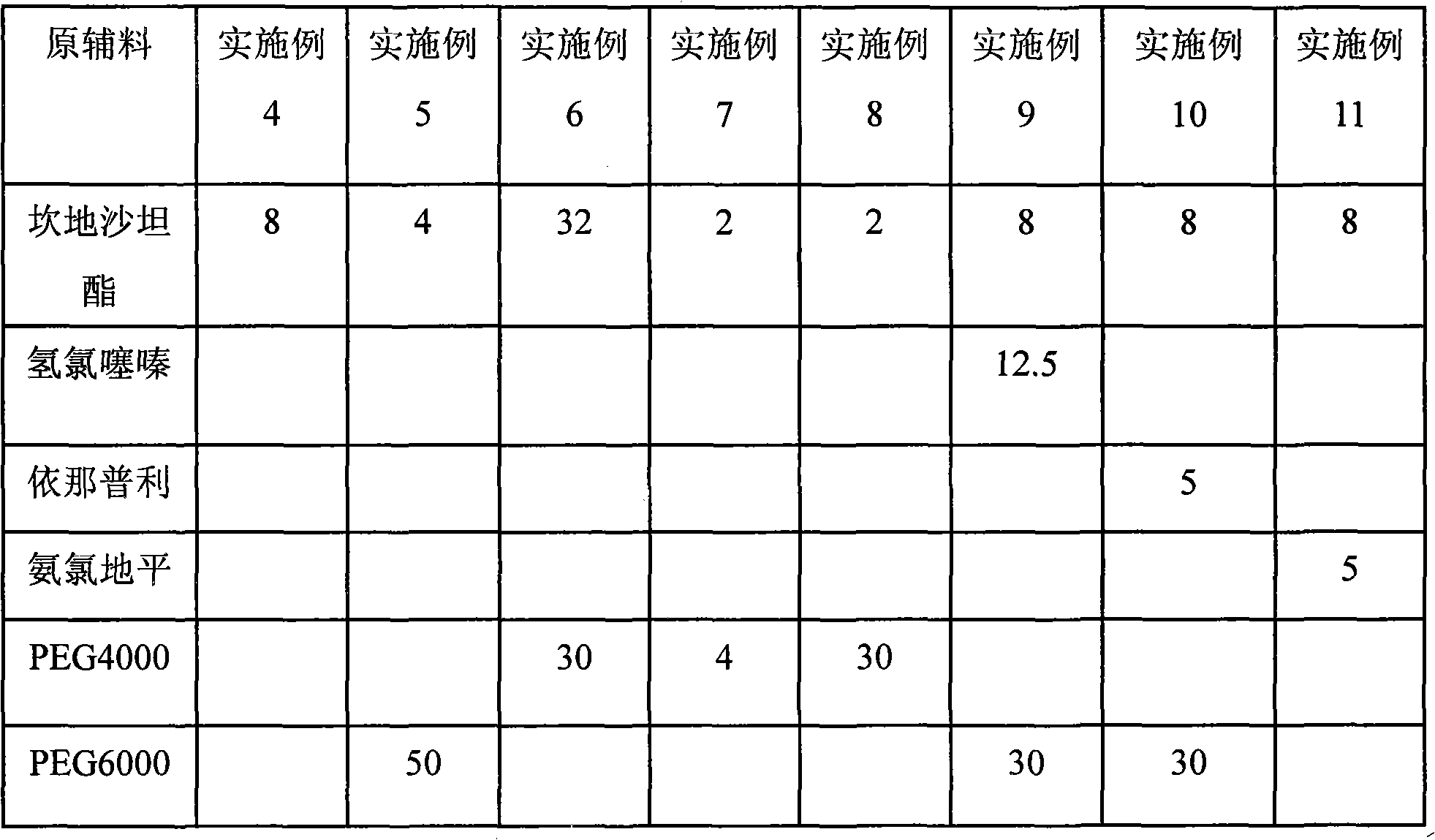
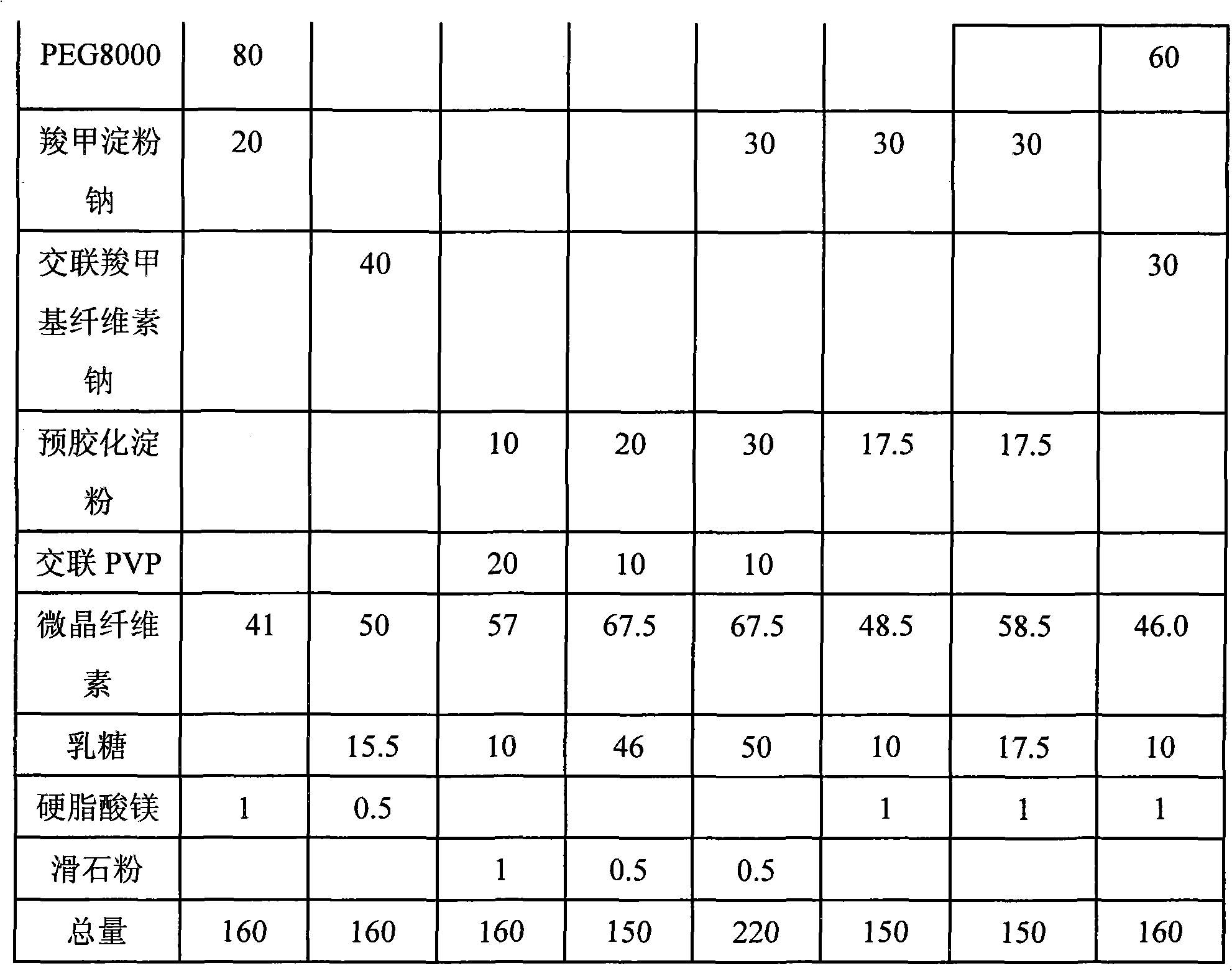
Examples
Embodiment 1
[0033] Candesartan cilexetil 4.0mg
[0034] PEG 4000 15.0mg
[0035] Microcrystalline Cellulose 65.0mg
[0036] Pregelatinized starch 40.0mg
[0037] Sodium starch glycolate 25.5mg
[0038]Magnesium stearate 0.5mg
[0039]
[0040] Total 150.0mg
[0041] Preparation process: take the prescription amount of raw and auxiliary materials, mix well, wet granulate, dry at 50 degrees, and fill HPMC capsules.
[0042] Example 1 comparison
[0043] The prescription and preparation process are the same as in Example 1, only the capsule shell used is a gelatin capsule shell.
Embodiment 2
[0045] Candesartan cilexetil 10.0mg
[0046] Lactose 84.0mg
[0047] Corn starch 20.0mg
[0048] PEG 6000 6.0mg
[0049] Hydroxypropyl Cellulose 4.0
[0050] Carmellose Calcium 5.6mg
[0051] Magnesium stearate 0.4mg
[0052]
[0053] Total 130.0mg
[0054] Preparation process: In a granulator equipped with a stirrer, the prescribed amount of candesartan cilexetil, hydrochlorothiazide, PEG 6000, and water are mixed, the mixture is added to other auxiliary material mixtures, granulated and dried to obtain granules, and then Add carmellose calcium and magnesium stearate. The resulting mixture was filled with hydroxypropylmethylcellulose (HPMC) capsules.
[0055] Example 2 comparison
[0056] The prescription and preparation method are the same as in Example 2, only the capsule shell used is a gelatin capsule shell.
Embodiment 3
[0058] Candesartan cilexetil 10.0mg
[0059] Hydrochlorothiazide 12.5mg
[0060] Lactose 71.5mg
[0061] Corn starch 20.0mg
[0062] PEG 6000 6.0 mg
[0063] Hydroxypropyl Cellulose 4.0mg
[0064] Carmellose Calcium 5.6mg
[0065] Magnesium stearate 0.4mg
[0066] (water) 0.024ml
[0067]
[0068] Total 130.0mg
[0069] Preparation process: In a granulator equipped with a stirrer, the prescribed amount of candesartan cilexetil, hydrochlorothiazide, PEG 6000, and water are mixed, the mixture is added to other auxiliary material mixtures, granulated and dried to obtain granules, and then Add carmellose calcium and magnesium stearate. The resulting mixture was filled with hydroxypropylmethylcellulose (HPMC) capsules.
[0070] Example 3 comparison
[0071] The prescription and preparation method are the same as in Example 3, only the capsule shell used is a gelatin capsule shell.
[0072] The hydroxypropylmethylcellulose (HPM...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com