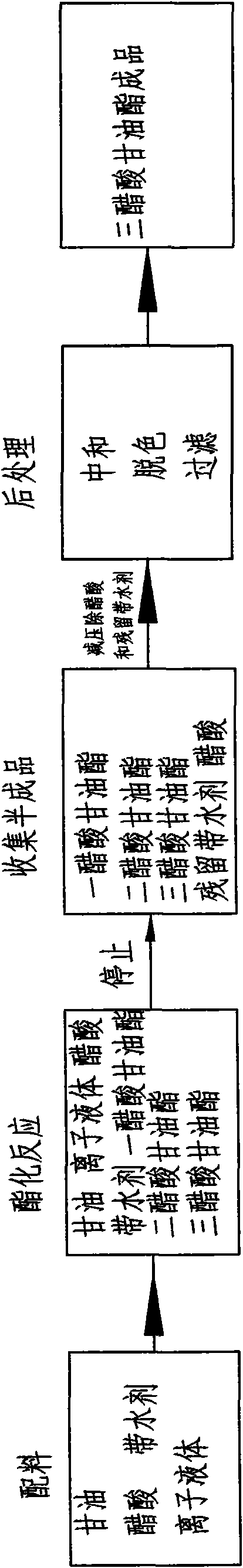
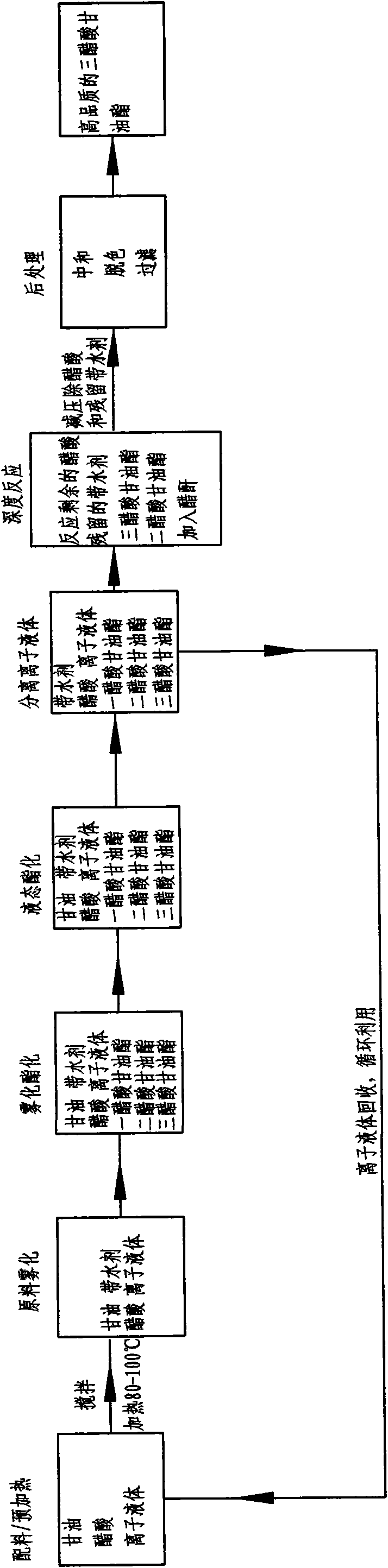
Method for producing glyceryl triacetate by atomizing raw materials
A technology of glycerol triacetate and raw materials, which is applied in chemical instruments and methods, chemical methods for reacting liquids with liquids, preparation of carboxylic acid esters, etc., can solve the problems of many side reactions, large energy loss, high reaction temperature, etc. Achieve the effect of shortening the esterification reaction time, complete the esterification reaction and improving the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In this example, ionic liquid [HSO3-pmim][HSO4] is used to catalyze the synthesis of glycerol triacetate, and the implementation method is as follows:
[0025] A, glycerin, acetic acid, liquid catalyst and water-carrying agent are matched in proportion, wherein by molar ratio, n (glycerol): n (acetic acid)=1: 8, and liquid catalyst accounts for 2.5% of reactant gross weight, and water-carrying The agent accounts for 4% of the total weight of the reactants, and the mixture is stirred and preheated to 90°C;
[0026] B. atomize the mixture obtained in step A, make it undergo esterification reaction at a temperature of 120°C for 2 hours, and obtain a liquid mixture with a triacetin content of 98%;
[0027] C, adding 1.5% acetic anhydride to the liquid mixture obtained in step B to carry out a deep esterification reaction, the reaction temperature is 110 ° C, and the reaction time is 20 minutes to obtain a liquid mixture with a triacetin content of 99.7%;
[0028] D. The se...
Embodiment 2
[0031] In this example, ionic liquid [HSO3-pmim][HSO4] is used to catalyze the synthesis of glycerol triacetate, and the implementation method is as follows:
[0032] A, glycerin, acetic acid, liquid catalyst and water-carrying agent are matched in proportion, wherein in molar ratio, n (glycerin): n (acetic acid)=1: 8, and liquid catalyst accounts for 4% of reactant gross weight, and water-carrying The agent accounts for 5% of the total weight of the reactants, and the mixture is stirred and preheated to 100°C;
[0033] B. atomize the mixture obtained in step A, and make it undergo esterification reaction at a temperature of 110°C for 1.5 hours to obtain a liquid mixture with a triacetin content of 98.5%;
[0034] C, the mixture obtained in step B is cooled to 30° C., and the liquid catalyst is layered and recovered;
[0035] D, adding 1.3% acetic anhydride to the liquid mixture obtained in step C to carry out a deep esterification reaction, the reaction temperature is 100° C...
Embodiment 3
[0039] In this example, ionic liquid [HSO3-pmim][HSO4] is used to catalyze the synthesis of glycerol triacetate, and the implementation method is as follows:
[0040] A, glycerin, acetic acid, liquid catalyst and water-carrying agent are matched in proportion, wherein in molar ratio, n (glycerin): n (acetic acid)=1: 8, and liquid catalyst accounts for 5% of reactant gross weight, and water-carrying The agent accounts for 6% of the total weight of the reactants, and the mixture is stirred and preheated to 95°C;
[0041] B. The mixture obtained in step A was atomized to allow esterification reaction to occur at a temperature of 105°C for 1.5 hours to obtain a liquid mixture with a triacetin content of 98.7%;
[0042] C, further reacting the liquid mixture obtained in step B for 1 hour at a temperature of 85° C. to obtain a liquid semi-finished product with a triacetin content of 98.8%;
[0043]D, adding 1% acetic anhydride to the liquid mixture obtained in step C to carry out a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com