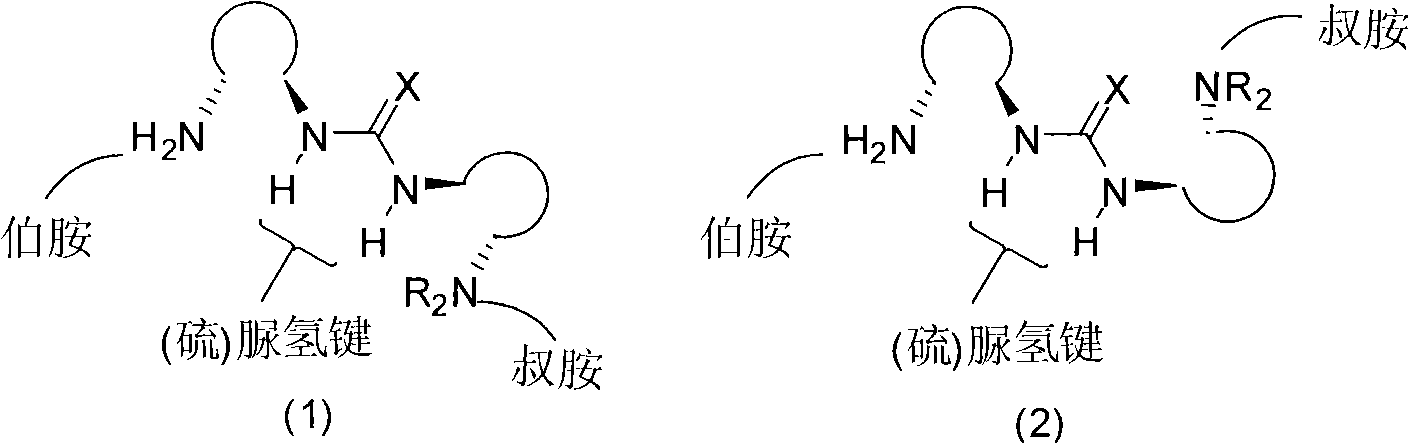
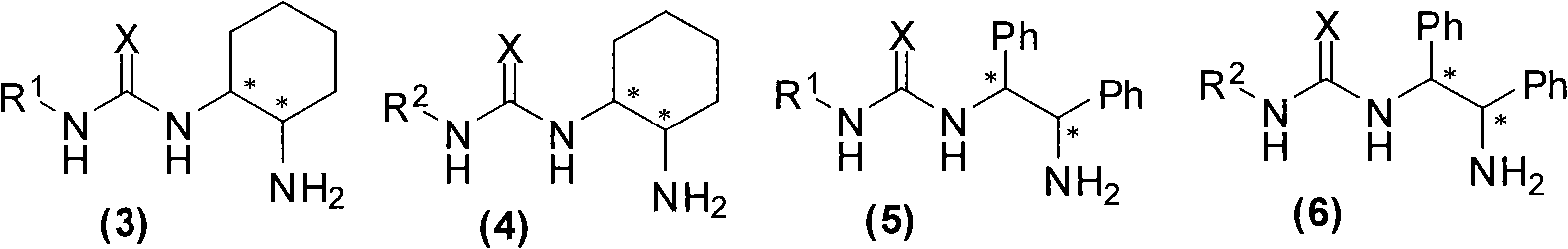
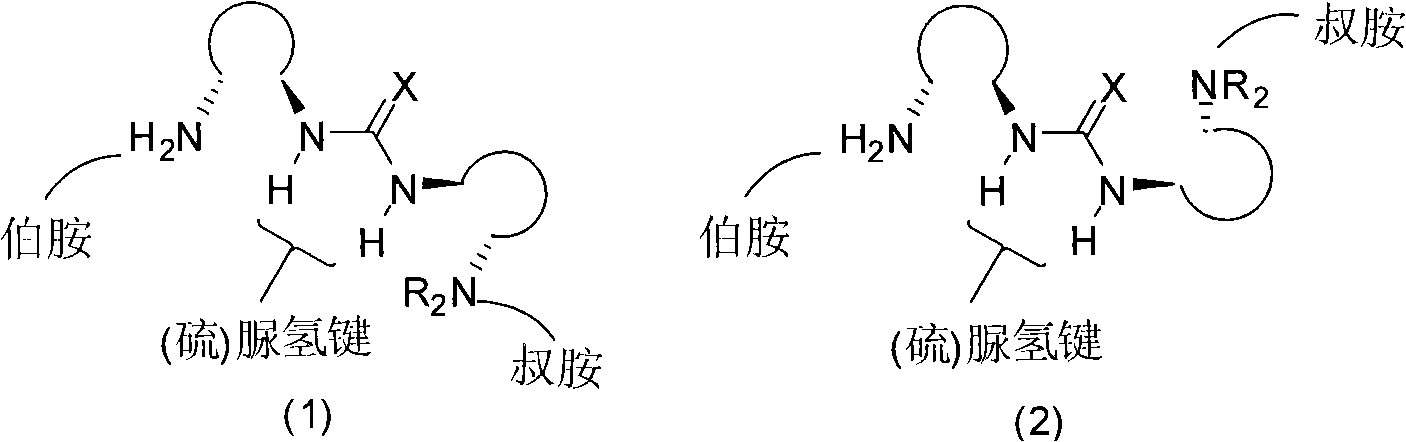
Organic catalyst containing primary amine, tertiary amine and urea or thiourea and preparation method thereof
A technology of organic catalyst and organic solvent, applied in the field of organic catalysis, to achieve the effect of unique structure, novel structure and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Under argon protection, (1R,2R)-1,2-cyclohexanediamine (6.85g, 0.06mol) and dichloromethane (60mL) were added to a 250mL flask, cooled to 0°C in an ice-water bath, and added dropwise with Boc 2 A solution of O (4.37 g, 0.02 mol) in dichloromethane (50 mL). The addition was completed within 30 minutes, the ice-water bath was removed, the temperature was naturally raised to room temperature, and the reaction was carried out at room temperature for 24 hours. Add water (30mL) to dissolve the white solid, extract with dichloromethane (25mL×3), dry, and concentrate to dryness under reduced pressure, add water (80mL), adjust the pH value to about 5 with 4M concentrated hydrochloric acid, add diethyl ether ( 50mL) extraction, standing still, liquid separation, the aqueous phase was extracted with diethyl ether (20mL×3), the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain (1R, 2R)-2-aminocyclohexylam...
Embodiment 2
[0043] Cinchonidine (2.94g, 10.0mmol) and triphenylphosphine (3.15g, 12.0mmol) were dissolved in freshly distilled THF (50mL), and cooled to 0°C in an ice-water bath. Under stirring, diisopropyl azodicarboxylate (2.43 g, 12.0 mmol) was added rapidly in one portion. Maintaining at 0°C, freshly distilled THF (20 mL) containing diphenylphosphoryl azide (DPPA, 3.30 g, 12.0 mmol) was added dropwise to the above reaction solution. After the dropwise addition, the reaction solution was naturally warmed to room temperature, reacted for 12 hours, and then heated to 50° C. for 2 hours. Additional triphenylphosphine (3.41 g, 13.0 mmol) was added and heating was maintained until no further gas evolution occurred (ca. 2h). After the reaction, the reaction solution was cooled to room temperature, water (1 mL) was added, stirred for 3 h, and then concentrated in vacuo to remove the solvent. Add dichloromethane-10% hydrochloric acid (1:1, 100mL) to dissolve the residue, let it stand and sep...
Embodiment 3
[0047] Under stirring, THF (40mL) dissolved with RBCS (3.07g, 12.0mmol) was added dropwise to freshly distilled THF (20mL) dissolved with 9-amino cinchonidine (2.93g, 10.0mmol). Stir overnight. After the reaction was completed, the solvent was removed by vacuum concentration, and the residue was separated and purified by silica gel column chromatography to obtain RBCD (4.67 g), a precursor of a multifunctional organic catalyst, with a yield of 85%.
[0048]
[0049] Under stirring, RBCD (2.75 g, 5.0 mmol) was dissolved in 1,4-dioxane (100 mL), and 4M hydrochloric acid (100 mL) was added to react at room temperature for 8 h. Concentrate in vacuo to dryness, add water (100mL) to dissolve, adjust the pH value in the range of 13-14 with 1M sodium hydroxide, a large amount of white suspended matter appears, extract with dichloromethane (4×50mL), combine the organic phases, and dry Dry over sodium sulfate and concentrate in vacuo to obtain RNCD (2.1 g) with a yield of 93%.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com