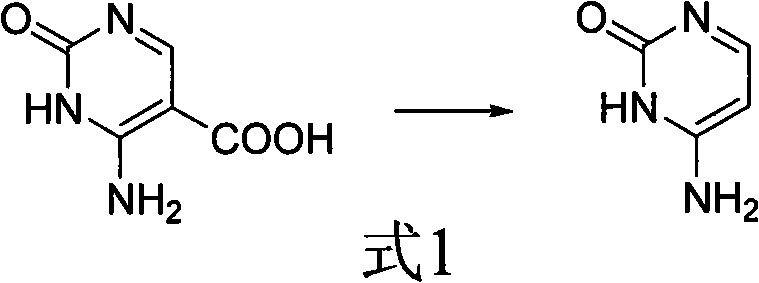
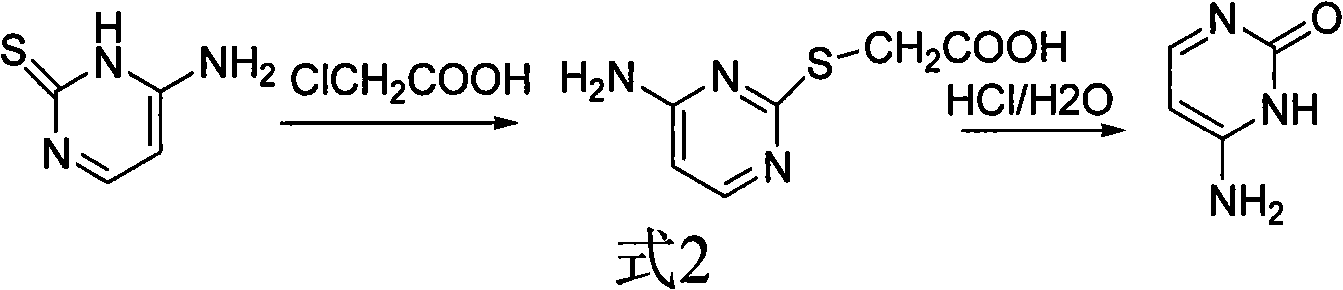
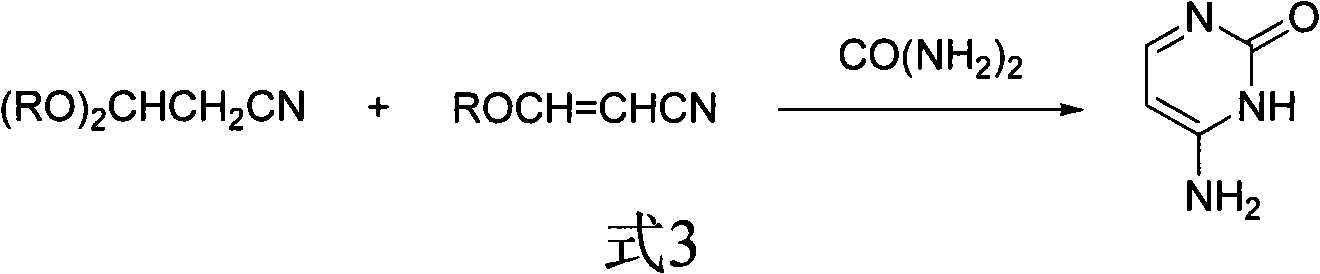Method for synthesizing cytimidine
A technology of cytosine and reactants, which is applied in the field of catalytic synthesis of cytosine, can solve the problems of complex reaction, expensive raw materials, and low yield, and achieve the effects of increasing reaction yield, shortening reaction time, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In a reaction flask, add 100g of 3-ethoxyacrylonitrile, 300mL of toluene, 200g of sodium methoxide (3.6:1 molar ratio with 3-ethoxyacrylonitrile), 94g of urea (with 3-ethoxyacrylonitrile The molar ratio is 1.5:1), acidic alumina 5g. The reaction mixture was reacted at 70°C, followed by HPLC to stop the reaction after the disappearance of 3-ethoxyacrylonitrile, cooled, added acetic acid to neutralize to pH=7, filtered to obtain a solid mixture, recrystallized with 200g of acetic acid, and recovered the catalyst , to obtain cytosine: mass 90.2g, melting point>280°C, content 99.3% (HPLC, area normalization method), yield 83% (calculated as 3-ethoxyacrylonitrile).
Embodiment 2
[0023] In a reaction flask, add 100g of 3-ethoxyacrylonitrile, 300mL of toluene, 200g of sodium methoxide (3.6:1 molar ratio with 3-ethoxyacrylonitrile), 100g of urea (with 3-ethoxyacrylonitrile The molar ratio is 1.6:1), 0.5 g of acidic alumina, and the reaction mixture was reacted at 70° C., followed by HPLC to stop the reaction after the disappearance of 3-ethoxyacrylonitrile, cooled, added acetic acid to neutralize to pH=7, Filtrate to obtain a solid mixture, recrystallize with 200g of acetic acid, recycle the catalyst, and obtain cytosine: 67g in quality, melting point > 280°C, content 99.3% (HPLC, area normalization method), yield 61.6% (based on 3-ethoxy Acrylonitrile meter).
Embodiment 3
[0025] In a reaction flask, add 100g of 3-ethoxyacrylonitrile, 300mL of toluene, 200g of sodium methoxide (3.6:1 molar ratio with 3-ethoxyacrylonitrile), 96g of urea (with 3-ethoxyacrylonitrile The molar ratio is 1.55:1), 5 g of 3A molecular sieves, the reaction mixture was reacted at 60 ° C, and the reaction was stopped after the disappearance of 3-ethoxyacrylonitrile by HPLC tracking detection, cooling, adding acetic acid to neutralize to pH = 7, and filtering. Obtain a solid mixture, recrystallize with 200 g of acetic acid, recycle the catalyst, and obtain cytosine: quality 98.3 g, melting point > 280 ° C, content 99.3% (HPLC, area normalization method), yield 90.5% (by 3-ethoxypropene Nitrile meter).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com