Prepartion method of CdSe and CdSe-ZnSe nuclear shell quantum dots
A technology of core-shell quantum dots and quantum dots, which is applied in the field of preparation of CdSe and CdSe-ZnSe core-shell quantum dots, can solve the problems of reduced fluorescent performance of quantum dots, unfavorable industrial production, cumbersome operation steps, etc., and achieves easy large-scale preparation , The preparation process is safe and reliable, and the fluorescence effect is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
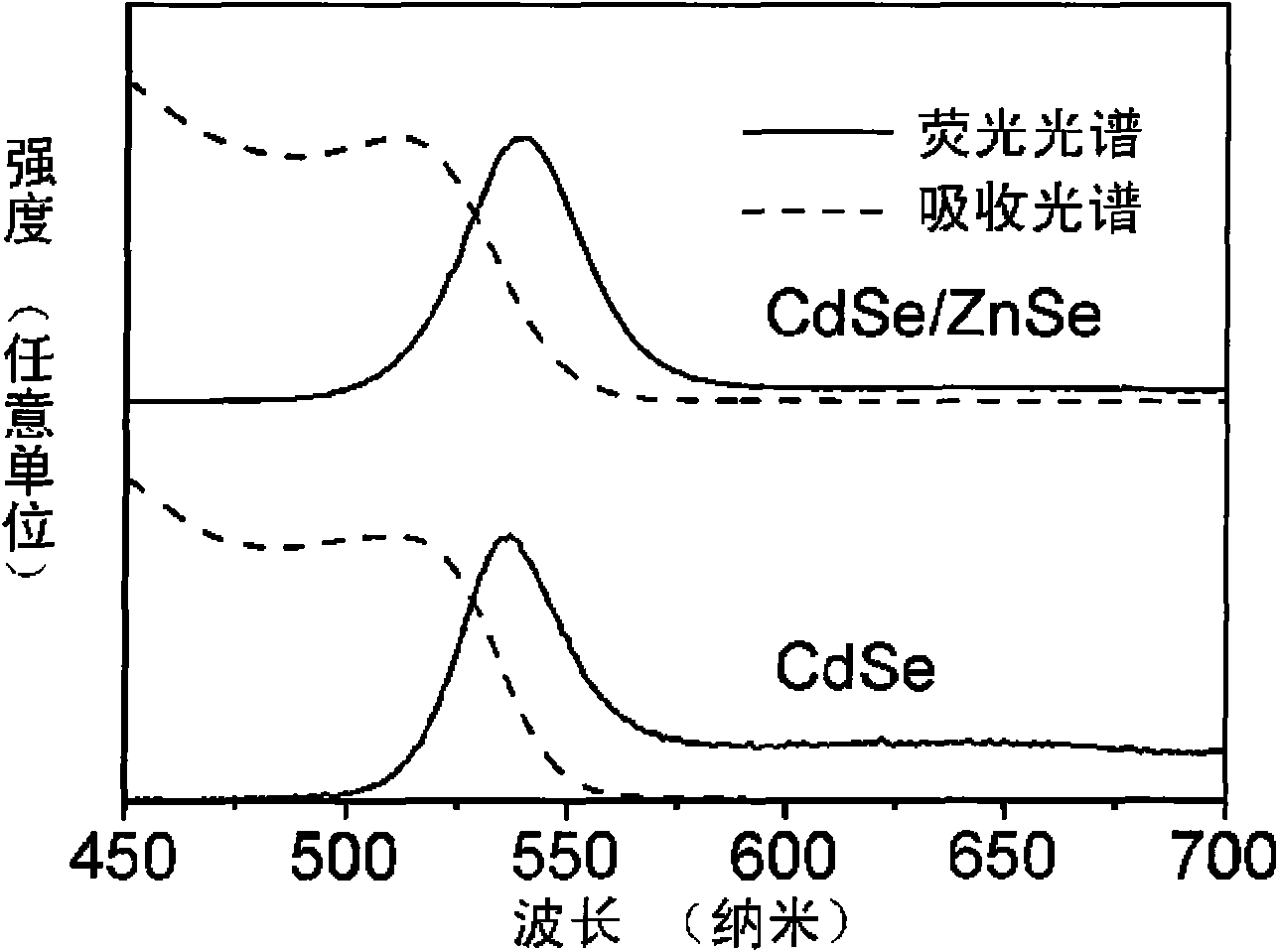
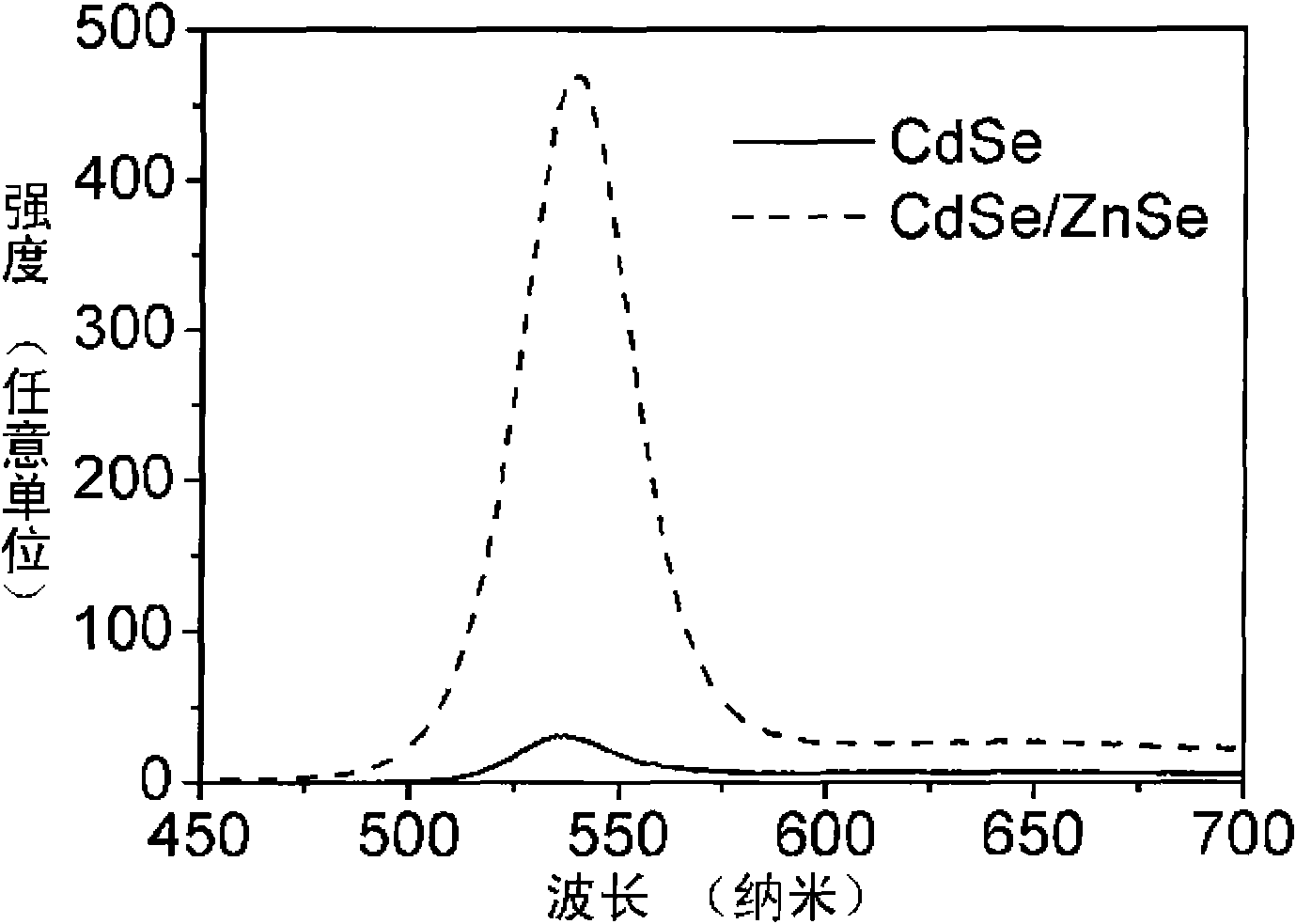
[0014] exist figure 1 and figure 2 In, 0.2mmol Cd(MA) 2 (cadmium myristate), 0.8mmol oleic acid and 10mL toluene were added to the reaction kettle, heated in an oven at 80°C until a colorless and transparent solution was obtained, and cooled to room temperature to obtain a Cd precursor solution; 0.2mmol Se powder, 0.36 ml trioctylphosphine (TOP) was added to 10mL toluene solution, and the Se powder was completely dissolved by ultrasonic at room temperature to obtain the Se precursor solution; the Cd precursor solution and the Se precursor solution were evenly mixed, and the N 2 After 10 minutes, seal it in the reactor, heat it in an oven at 220° C. for 1.7 h, and cool the reactor after heating to room temperature in the air to obtain CdSe quantum dots with different emission wavelengths.
[0015] 0.0295g Zn(MA) 2 , 0.072mL oleic acid and 22.2mL toluene were added to the reaction kettle, heated in an oven at 100°C until completely dissolved, and then cooled to room temperat...
Embodiment 2
[0017] 0.4mmol Cd(MA)2 (cadmium myristate), 0.8mmol oleic acid and 10mL octadecene were added to the reaction kettle, heated in an oven at 100°C until a colorless and transparent solution was obtained, and cooled to room temperature to obtain a Cd precursor solution; 0.2mmol Se powder , 0.36ml trioctylphosphine (TOP) was added to 10mL octadecene solution, and the Se powder was completely dissolved by ultrasonic at room temperature to obtain the Se precursor solution; the Cd precursor solution was uniformly mixed with the Se precursor solution, and the N 2 After 10 minutes, seal it in the reaction kettle, heat it in an oven at 220°C for 1.7, and cool the reaction kettle with the heating off to room temperature in the air to obtain CdSe quantum dots.
[0018] 0.1379g Zn(MA) 2 , 0.34mL oleic acid and 22.2mL octadecene were added to the reaction kettle, heated in an oven at 140°C until completely dissolved, and then cooled to room temperature to obtain a Zn precursor solution; the...
Embodiment 3
[0020] 0.1mmol Cd(MA) 2 (cadmium myristate), 0.8mmol oleic acid and 10mL toluene were added to the reaction kettle, heated in an oven at 90°C until a colorless and transparent solution was obtained, and cooled to room temperature to obtain a Cd precursor solution; 1.0mmol Se powder, 0.36 ml trioctylphosphine (TOP) was added to 10mL toluene solution, and the Se powder was completely dissolved by ultrasonic at room temperature to obtain the Se precursor solution; the Cd precursor solution and the Se precursor solution were evenly mixed, and the N 2 After 10 minutes, it was sealed in a reaction kettle, heated in an oven at 220° C. for 1.7 hours, and the heated reaction kettle was cooled to room temperature in air to obtain CdSe quantum dots.
[0021] 0.0743g Zn(MA) 2 , 0.18mL oleic acid and 22.2mL toluene were added to the reaction kettle, heated in an oven at 120°C until completely dissolved, and then cooled to room temperature to obtain a Zn precursor solution; then added Se p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com