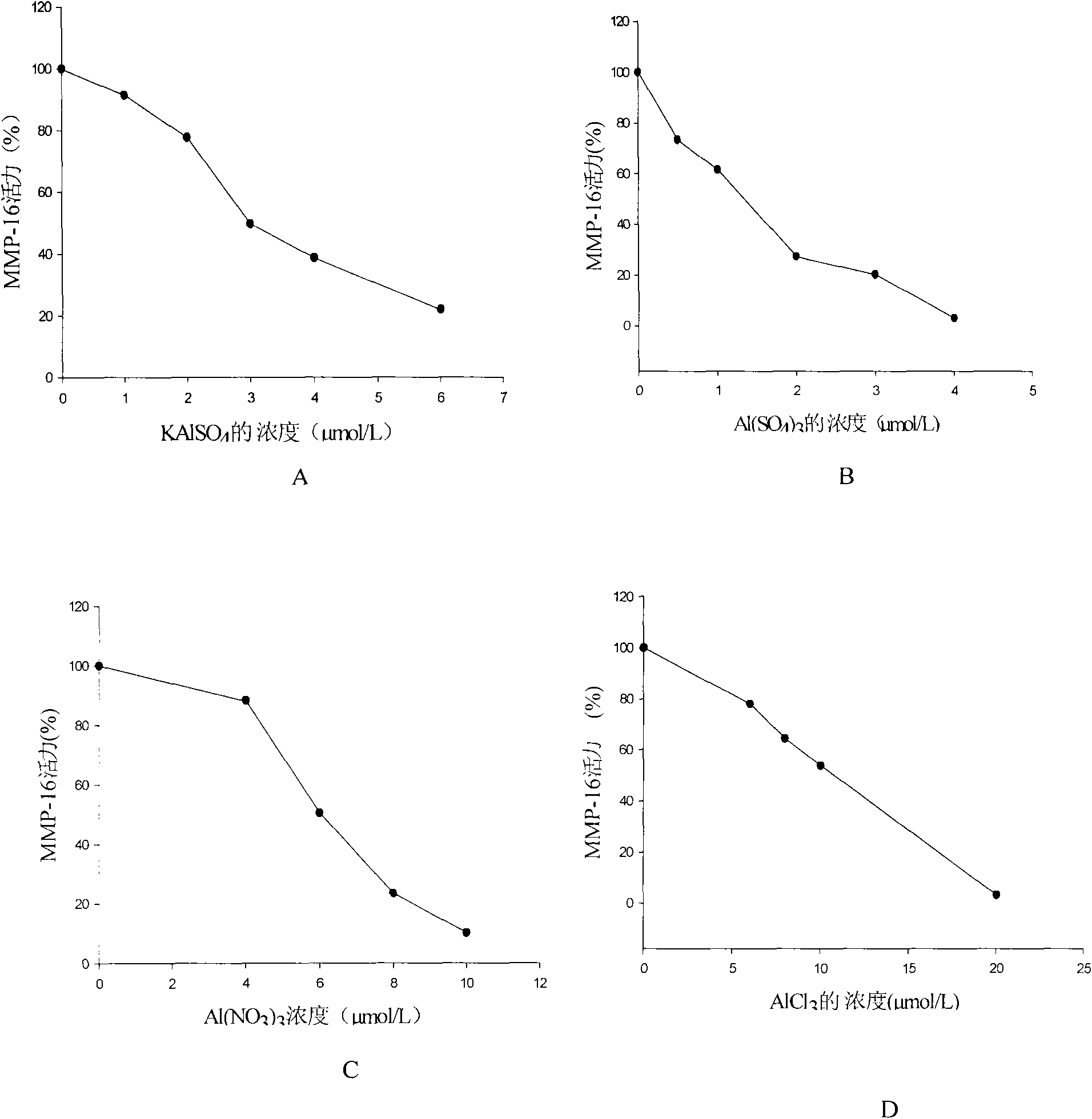Anti-tumor-stroma metalloprotease inhibitor
A technology of protease inhibitors and matrix metals, applied in the field of biomedicine, can solve the problems of reducing the selective inhibitory ability of MMPs, and achieve the effects of strong MMPs inhibitory activity, low price, and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Inhibitory effect of aluminum ammonium sulfate on MMP-16: The reaction was carried out in 50mmol / L HEPES buffer system (pH6.8), which contained 200mmol / L NaCl, 10mmol / LCaCl 2 , 20μmol / LZnCl 2 , 10mmol / L MgCl 2 and 0.01% Brij-35. At a certain enzyme concentration, ammonium aluminum sulfate was added to the reaction mixture at different concentrations, placed at 37°C for 30 minutes, and the reaction was initiated by adding 1 μL of 200 μg / ml DQ-gelatin substrate. The inhibition efficiency was determined by comparing the enzyme activity in the presence or absence of the inhibitor. The fluorescence was detected by FLX800 fluorescence microplate reader (Bio-Tek). The excitation wavelength was at 495 nm and the emission wavelength was at 515 nm. All experiments were performed in 96-well plates, and each measurement was corrected for background fluorescence absorption subtracted. It was found that ammonium aluminum sulfate has a good inhibitory effect on MMP-16, and its IC ...
Embodiment 2
[0043] Inhibitory effect of potassium aluminum sulfate on MMP-16: The reaction was carried out in 50mmol / L HEPES buffer system (pH 6.8), which contained 200mmol / L NaCl, 10mmol / LCaCl 2 , 20μmol / LZnCl 2 , 10mmol / L MgCl 2 and 0.01% Brij-35. At a certain concentration of enzyme concentration, potassium aluminum sulfate was added to the reaction mixture at different concentrations, placed at 37°C for 30 minutes, and the reaction was initiated by adding 1 μL of 200 μg / ml DQ-gelatin substrate. The inhibition efficiency was determined by comparing the enzyme activity in the presence or absence of the inhibitor, and the fluorescence was detected by a FLX800 fluorescence microplate reader (Bio-Tek). The excitation wavelength was at 495 nm and the emission wavelength was at 515 nm. All experiments were performed in 96-well plates, and each measurement was corrected for background fluorescence absorption subtracted. It was found that potassium aluminum sulfate has a good inhibitory ef...
Embodiment 3
[0045] Inhibitory effect of aluminum sulfate on MMP-16: The reaction was carried out in 50mmol / L HEPES buffer system (pH6.8), which contained 200mmol / L NaCl, 10mmol / LCaCl 2 , 20μmol / LZnCl 2 , 10mmol / L MgCl 2 and 0.01% Brij-35. At a certain concentration of enzyme concentration, aluminum sulfate was added to the reaction mixture at different concentrations and placed at 37°C for 30 minutes. The reaction was initiated by adding 1 μL of 200 μg / ml DQ-gelatin substrate. The inhibition efficiency was determined by comparing the enzyme activity in the presence or absence of the inhibitor. The fluorescence was detected by a FLX800 fluorescence microplate reader (Bio-Tek), and the excitation wavelength was 495 nm. , the emission wavelength is 515nm. All experiments were performed in 96-well plates, and each measurement was corrected for background fluorescence absorption subtracted. It was found that aluminum sulfate has a good inhibitory effect on MMP-16, and its IC 50 is 1.56 μm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com