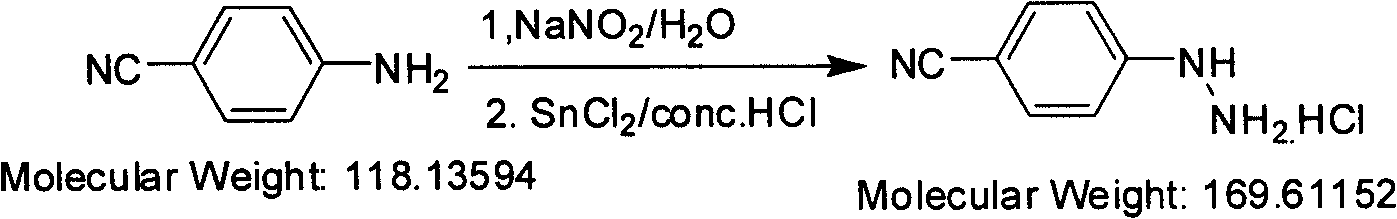Preparation method of paracyano-group phenyl hydrazine hydrochloride
A technology of cyanophenylhydrazine and hydrochloride, which is applied in the field of preparation of aromatic hydrazine hydrochloride synthesis, can solve problems such as low yield and poor crystal form, achieve short production time, low requirements for production equipment, The effect of high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Synthesis of p-cyanophenylhydrazine hydrochloride
[0013] Under mechanical stirring, (1kg, 8.5mol) p-cyanoaniline was dissolved in a 20-liter reactor of water (2.4L) and hydrochloric acid (3.1L), cooled to 5-10°C with an ice bath, and the reaction solution became White suspension. Slowly add sodium nitrite aqueous solution (602g / 5L) dropwise, and control the internal temperature at -5-5°C during the dropwise addition. After the dropwise addition, it can be observed that the reaction solution turns into a clear and transparent solution. After stirring for half an hour at -5-5°C, add SnCl dropwise to the clear solution 2 .2H 2 O (3.87kg, 16.9mol) of hydrochloric acid solution (35-37% HCl, 4.2L), the internal temperature is controlled at 5-10°C during the dropwise addition. The dropwise addition process was accompanied by heat release, and white solids were gradually precipitated. Stir at 5-10°C for 1 hour and filter. The filter cake was washed with 35-37% hydroch...
Embodiment 2
[0017] Synthesis of p-cyanophenylhydrazine hydrochloride
[0018] Under mechanical stirring, (1kg, 8.5mol) p-cyanoaniline was dissolved in a 20-liter reactor of water (2.4L) and hydrochloric acid (3.1L), cooled to 5-10°C with an ice bath, and the reaction solution became White suspension. Slowly add sodium nitrite aqueous solution (602g / 5L) dropwise, and control the internal temperature at -5-5°C during the dropwise addition. After the dropwise addition, it can be observed that the reaction solution turns into a clear and transparent solution. After stirring for half an hour at -5-5°C, add SnCl dropwise to the clear liquid 2 .2H 2 O (3.87kg, 16.9mol) of hydrochloric acid solution (35-37% HCl, 4.2L), the internal temperature is controlled at 5-10°C during the dropwise addition. The dropwise addition process was accompanied by heat release, and white solids were gradually precipitated. Stir at -5-5°C for 1 hour and filter. The filter cake was washed with 35-37% hydrochlo...
Embodiment 3
[0022] Synthesis of p-cyanophenylhydrazine hydrochloride
[0023] Under mechanical stirring, (1kg, 8.5mol) p-cyanoaniline was dissolved in a 20-liter reactor of water (2.4L) and hydrochloric acid (3.1L), cooled to 5-10°C with an ice bath, and the reaction solution became White suspension. Slowly add sodium nitrite aqueous solution (602g / 5L) dropwise, and control the internal temperature at -5-5°C during the dropwise addition. After the dropwise addition, it can be observed that the reaction solution turns into a clear and transparent solution. After stirring at 5-10°C for half an hour, add SnCl dropwise to the clear solution 2 .2H 2 O (3.87kg, 16.9mol) of hydrochloric acid solution (35-37% HCl, 4.2L), the internal temperature is controlled at 5-10°C during the dropwise addition. The dropwise addition process was accompanied by heat release, and white solids were gradually precipitated. Stir at -5-5°C for 1 hour and filter. The filter cake was washed with 35-37% hydroch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com