Aurone derivative-containing composition for diagnosis
A composition and compound technology, applied in the directions of preparations for in vivo experiments, organic chemistry, in vivo radioactive preparations, etc., can solve the problems of low permeability, no input, low clearance rate, etc., and achieve the effect of high binding specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] experimental method
[0092] (1) Reagents and instruments
[0093] As radioactive iodine-125 ( 125 I) using IODINE-125 (185 MBq) from Amersham Bioscience. For reverse-phase HPLC, a Cosmosil 5C18-AR column (4.6 x 150mm) from Nacalai Tesque was used, and ultrapure water (A) and acetonitrile (B) were used as eluents at a mixing ratio of A:B=30:70 , with a flow rate of 1.0 mL / min. Mass spectra were obtained on a JEOL IMS-DX instrument. Amyloid beta protein (Human, 1-42) [TFA form] was purchased from Peptide Institute. Other reagents are grade specific. Obtained on a Varian Gemini 300 spectrometer 1 H-NMR spectrum with tetramethylsilane as internal standard.
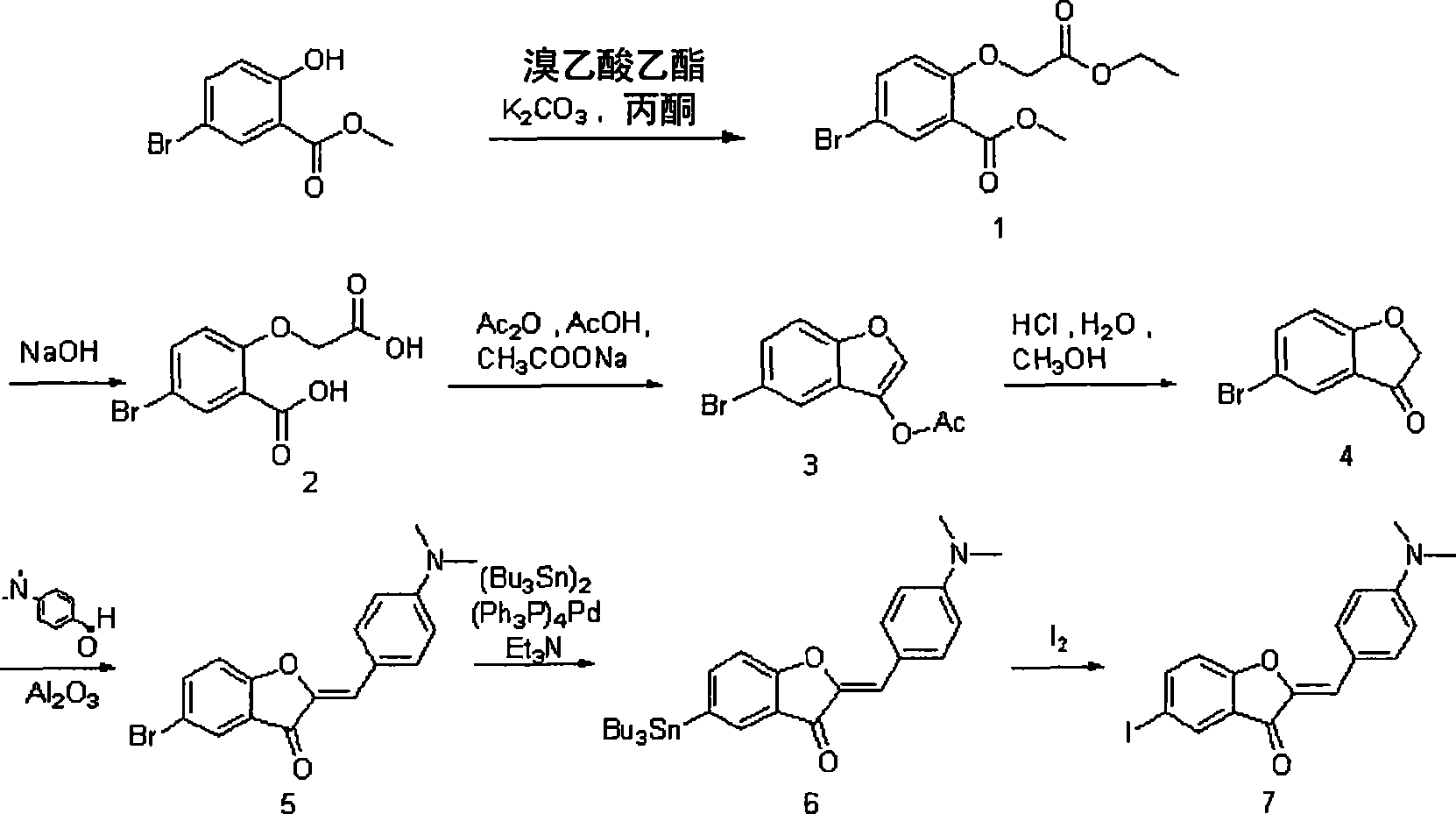
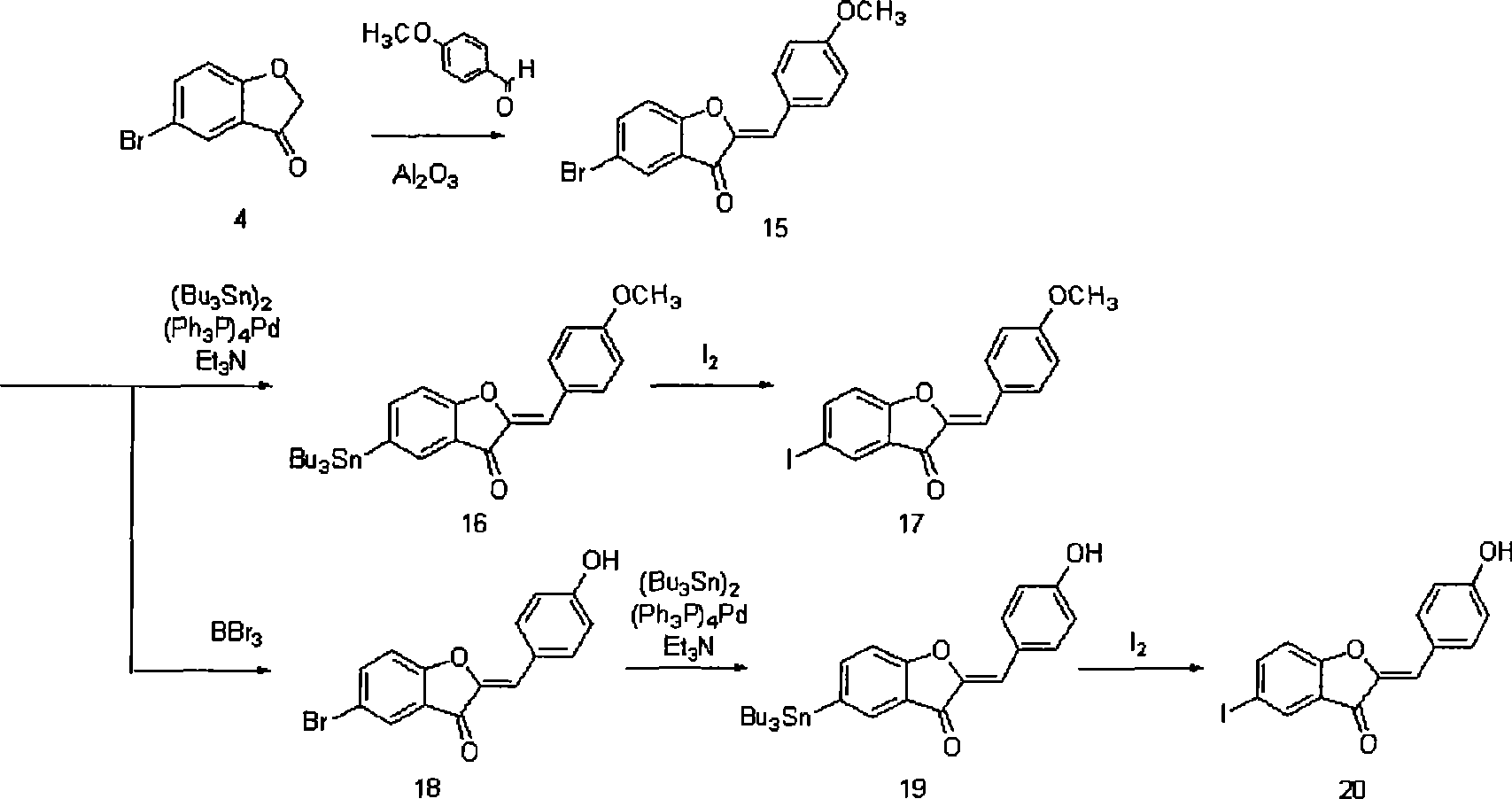
[0094] (2) Synthesis of 2-benzylbenzofuranone derivatives
[0095] Synthesis of methyl 2-(2-(methoxy-2-oxyethoxy)-5-bromobenzoate (compound 1)
[0096] To a solution of methyl 2-hydroxy-5-bromobenzoate (1.5 g, 6.49 mmol) in acetone (10 mL) was added K 2 CO 3 (2.7g). Ethyl bromoacetate (1.3 mL) was then adde...
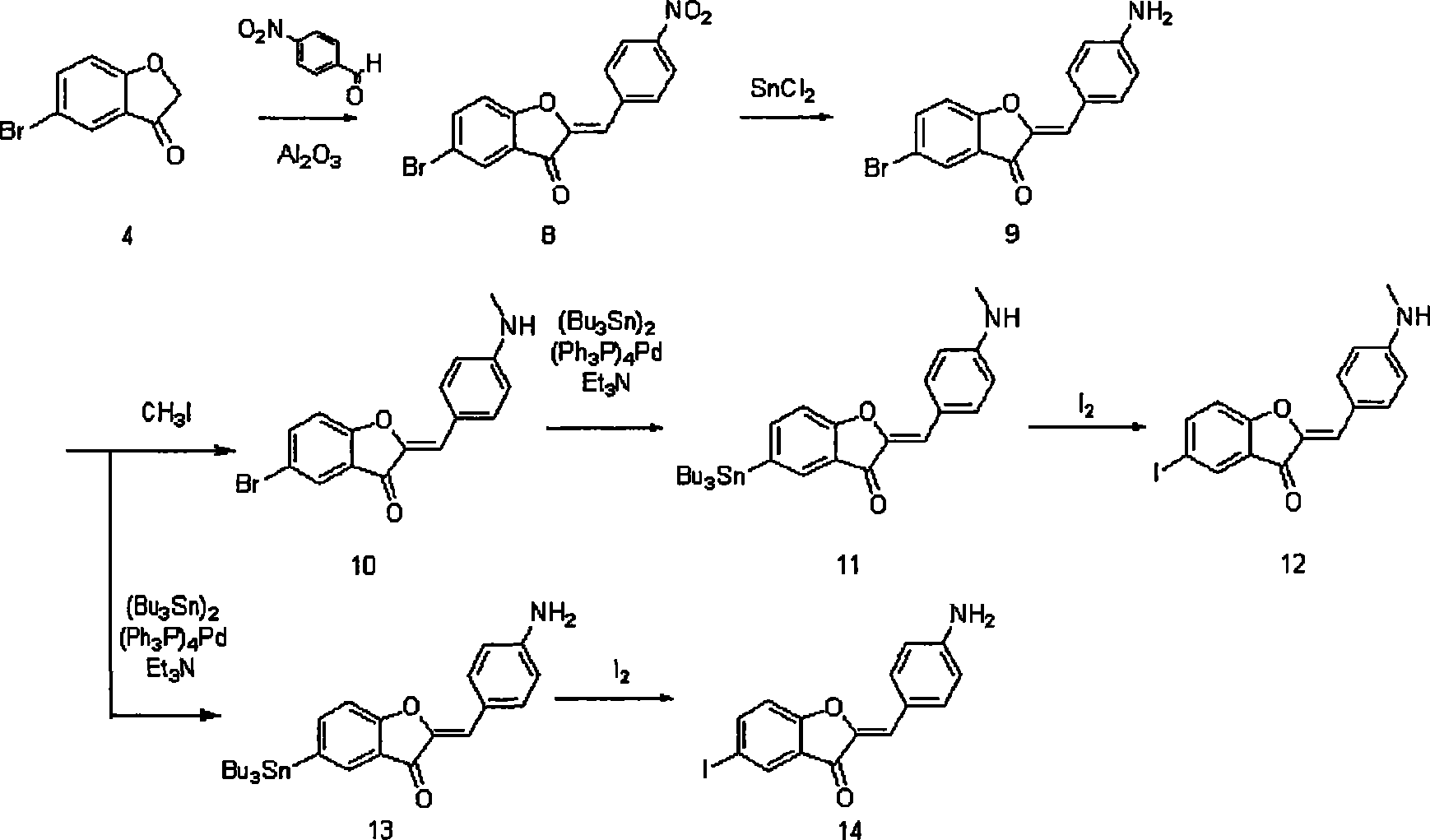
Embodiment 2
[0154] experimental method
[0155] (1) Reagents and instruments
[0156] The same reagents and equipment as in Example 1 were used.
[0157] (2) Synthesis of 2-benzylbenzofuranone derivatives
[0158] Synthesis of methyl 2-((ethoxycarbonyl)methoxy)-5-iodobenzoate (compound 21)
[0159] To a solution of methyl 2-hydroxy-5-iodobenzoate (2.0 g, 7.19 mmol) in acetone (10 mL) was added K 2 CO 3(2.7g). Subsequently, ethyl bromoacetate (2.0 mL) was added dropwise thereto with stirring. The mixture was heated to reflux for 3 hours. After the reaction was complete, the solvent was evaporated. The residue was dissolved in pure water (100 mL), followed by extraction with ethyl acetate (100 mL). The extract was dried over anhydrous sodium sulfate, and the solvent was evaporated to give compound 21. Yield: 2.62g (Yield: 99%) 1 H NMR (300MHz, CDCl 3 ) 1.29(t, J=7.2Hz, 3H), 3.90(s, 3H), 4.25(q, J=6.0Hz, 2H), 4.69(s, 2H), 6.66(d, J=8.7Hz, 1H ), 7.71 (dd, J=2.4, 2.4Hz, 1H), 8.12 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com