Anti-Alzheimer disease monoclonal antibody and application thereof
A technology for Alzheimer's disease and monoclonal antibodies, applied in the field of new anti-Alzheimer's disease monoclonal antibodies, can solve the problems of inability to specifically recognize Aβ oligomers, immunization methods and effects are not ideal, and achieve immune High serum titer, good immune effect, and the effect of reducing cerebral hemorrhage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
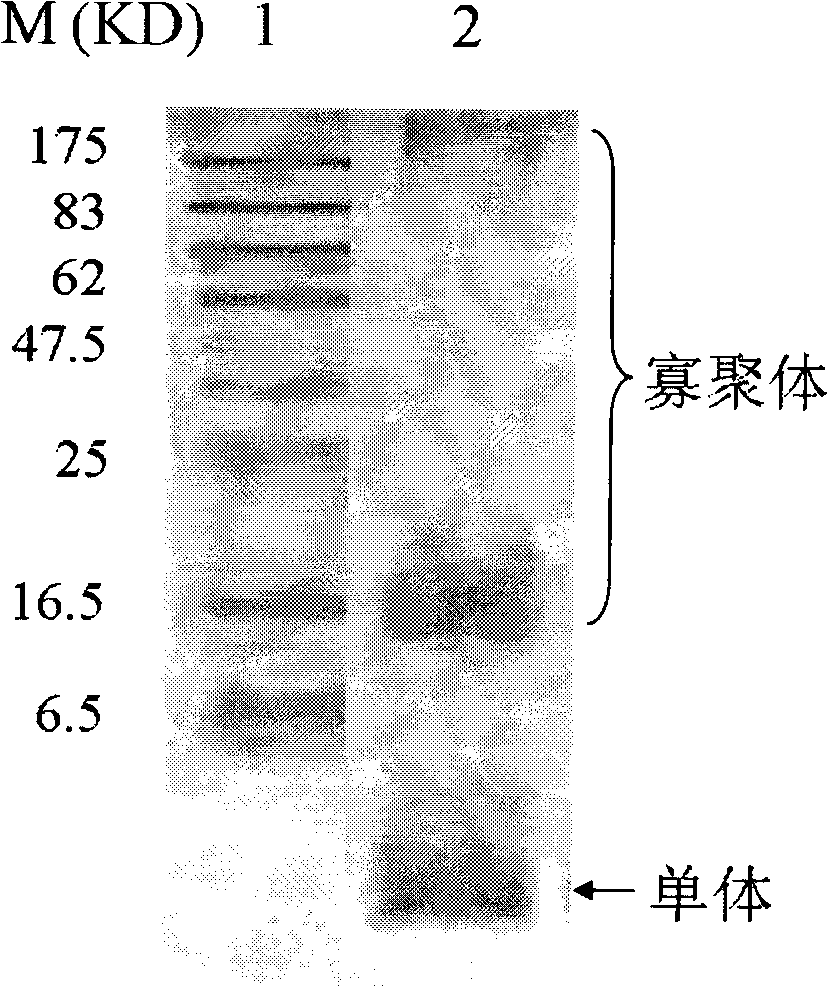
[0050] Example 1 Preparation of oligomer high affinity anti-human Aβ monoclonal antibody A8
[0051] 1. Preparation of Aβ Oligomerization Mixture
[0052] Aβ 1-42 The peptide was synthesized by Shanghai Sangon Bioengineering Technology Service Co., Ltd. Refer to the literature (Lambert M, 1998) method, and make necessary adjustments, assemble it in vitro to obtain Aβ under similar natural conditions 1-42 Oligomeric mixture. Aβ 1-42 Peptide 1mg was dissolved in ice-cold hexafluoroisopropanol (1,1,1,3,3,3-hexafluoro-2-βroβannol, HFIP) (Sigma) to make Aβ 1-42 Peptide monomerization, room temperature, after 1h, the HFIP was completely evaporated. Then, 20 μl of anhydrous dimethyl sulfoxide (DMSO) (Sigma) was used to dissolve the Aβ1-42 monomer, and finally it was placed in F12 medium (Sigma) or phosphate buffer system (the volume was supplemented by 1 ml), and placed at 4°C. 24h, let it naturally aggregate, and use Western blot to detect Aβ 1-42 Preparation of oligomeric mi...
Embodiment 2
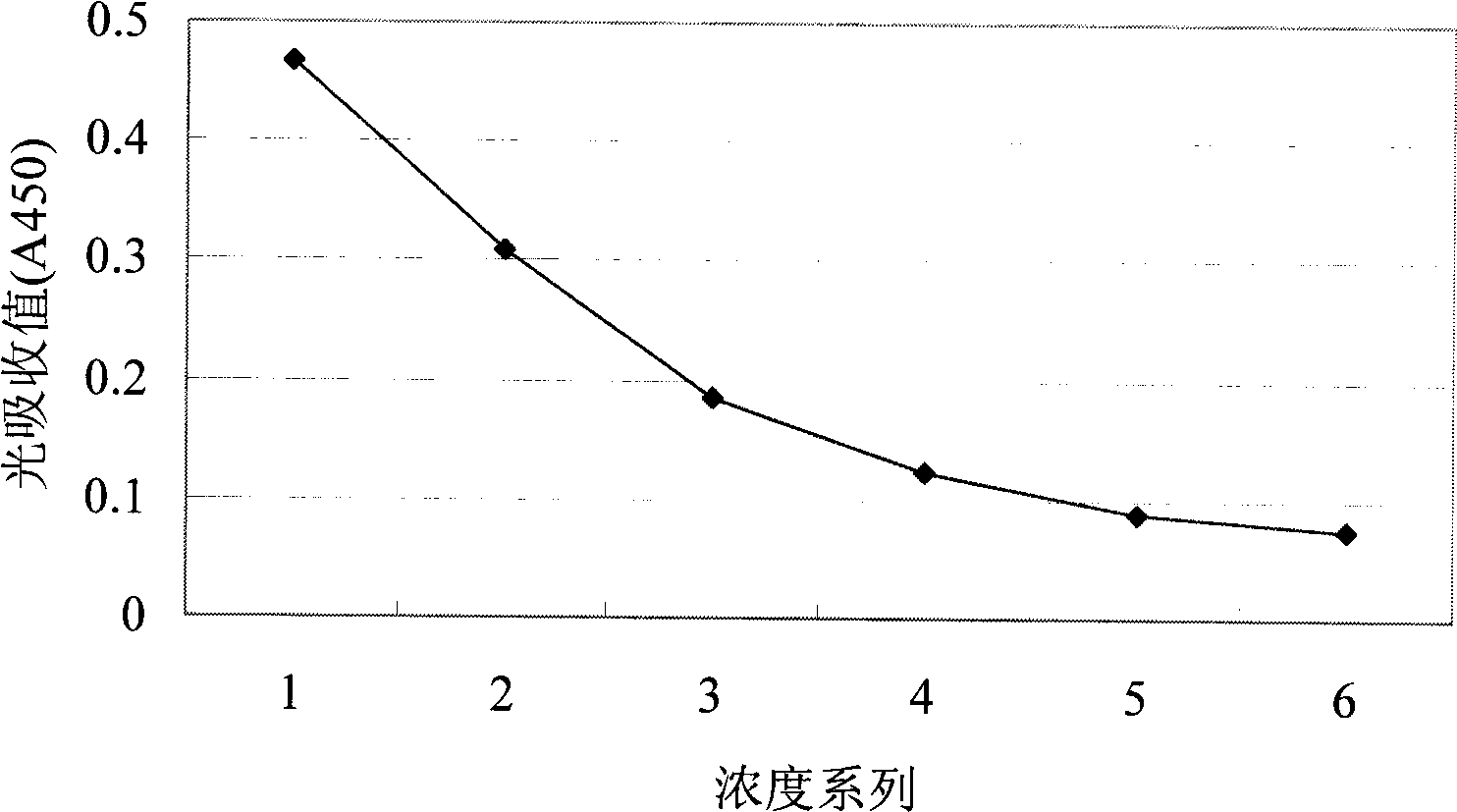
[0070] Example 2 Indirect ELISA experiment
[0071] 1. Method
[0072] (1) Coating: 10μg·ml -1 The Aβ oligomerization mixture is the coating antigen, which is added to a microtiter plate (SUNRISE Company), 100 μl per well, and left overnight at 4°C.
[0073] (2) PBS-T (NaCl 8g, KCl 0.2g, NaCl 2 HPO 4· 1.44g, KH 2 PO 4· 0.44g, Tween-20 0.05ml, add ddH 2 0 to 1L, pH7.2~7.4) Wash the plate: 3 times, 5min each time.
[0074] (3) Blocking: add PBS-T containing 0.2% BSA, 100 μl per well. 37°C, 2h.
[0075] (4) Add the double-diluted monoclonal antibody A8 into the 96-well plate, 100 μl per well. 37°C, 2h. At the same time, a blank control, a negative control and a positive control were set up (mouse anti-human Aβ serum and Calbiochem anti-human Aβ1-17 were used respectively).
[0076] (5) Plate washing with PBS-T: 3 times, 5 minutes each time.
[0077] (6) Add 1:10000 dilution of HRP-labeled goat anti-mouse IgG (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.), 100 μ...
Embodiment 3
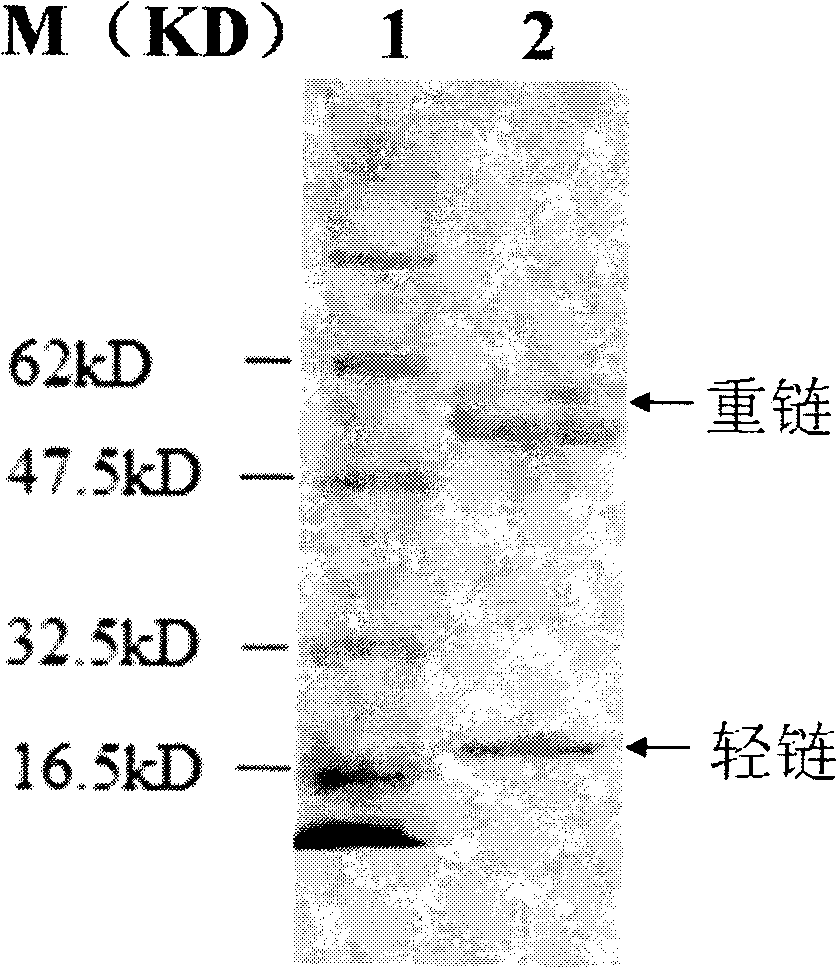
[0086] Example 3 Western blot (Western blot) experiment
[0087] 1. Method
[0088] Take 50-100μg sample, 5×sample buffer, mix well and load the sample, first make the protein pass through the stacking gel with a voltage of 100V. When the sample enters the separating gel, adjust the voltage to keep it constant at 120V. When the bromophenol blue swims to the bottom of the gel, end the electrophoresis, remove the gel, and stain it with Coomassie Brilliant Blue R-250 routinely; put the gel and nitrocellulose membrane into containers containing blotting buffer Equilibrate in the chamber for 10 minutes, put filter paper, gel, NC membrane, and filter paper in order to form a "sandwich" shape, pour the transfer buffer, with the gel side facing the negative electrode and the NC membrane facing the positive electrode, carefully avoiding and driving away air bubbles. Turn on the power, make the constant current 80mA transfer continuously for 2h, cut off the power.
[0089] After the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com