Composition for two-component fluorine coating material
A composition and coating technology, which is applied in the direction of anti-corrosion coatings, polyurea/polyurethane coatings, coatings, etc., can solve the problem of damage to old coating films, and achieve the effects of excellent physical properties, excellent processability, and excellent solubility of the coating film
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
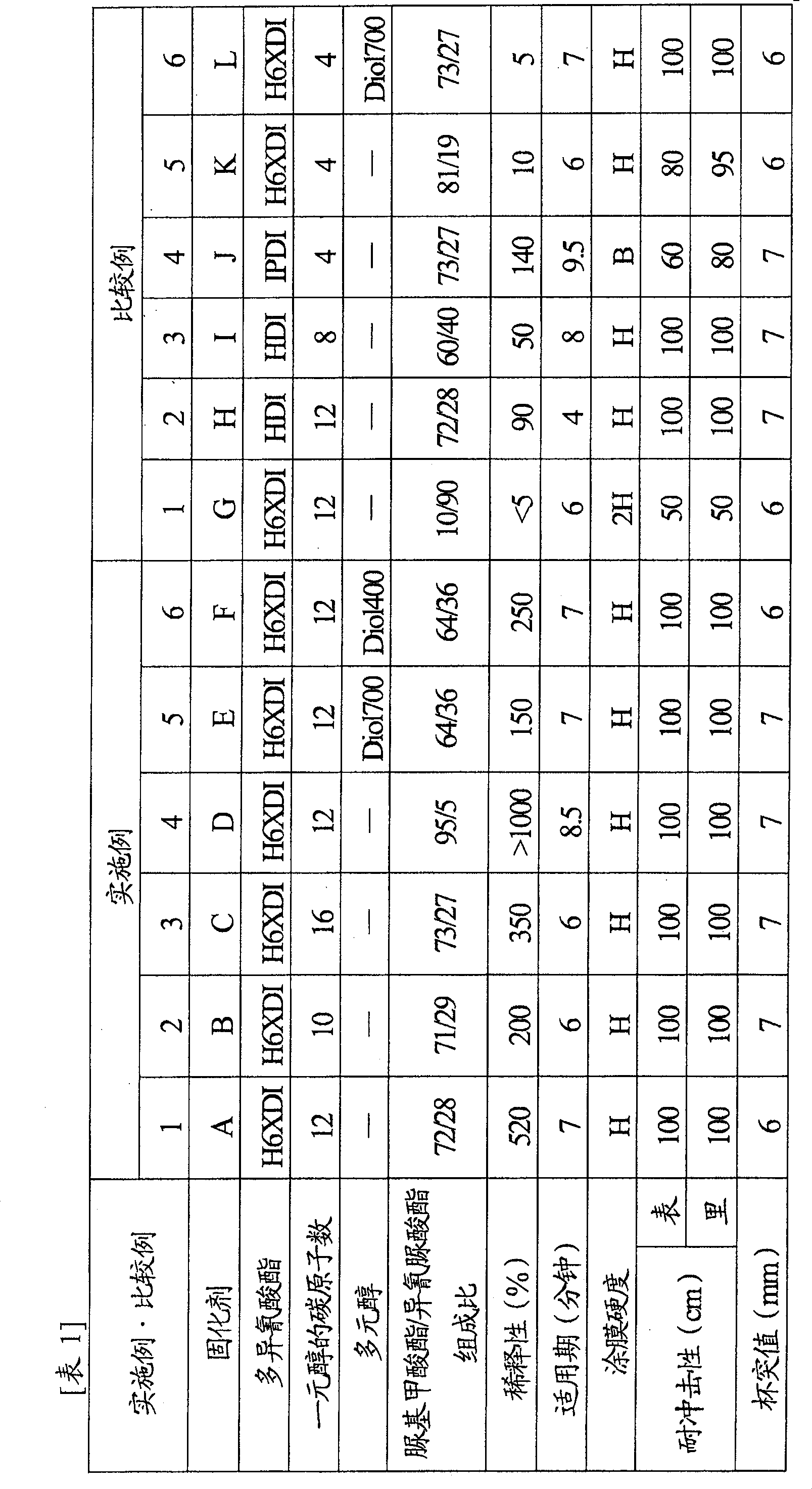
[0093] Next, although this invention is demonstrated based on an Example and a comparative example, this invention is not limited to a following example.
Synthetic example 1
[0094] Synthesis example 1 (synthesis of curing agent A)
[0095] Under nitrogen atmosphere, charge 455.6gH into a 500mL capacity four-necked bottle equipped with stirrer, thermometer, nitrogen inlet tube and Dimro reflux condenser (Dimroth condenser). 6 XDI and 44.4g dodecanol, heated to 90°C and held for 2 hours.
[0096] Afterwards, as a reaction catalyst, add 0.02g of 2-ethylhexanoic acid trimethyl-N-2-hydroxypropyl ammonium (trimethyl-N-2-hydroxypropyl ammonium2-ethylhexanoate), while adjusting the reaction temperature to 90±5°C, While continuing to react for 2 hours. Then, 0.02 g of o-toluenesulfonamide was added as a catalyst deactivator to deactivate the reaction catalyst and stop the reaction.
[0097] Remove unreacted H from the resulting reaction solution 6 XDI obtained 201.1 g of light yellow and transparent polyisocyanate curing agent A (conversion rate 40%).
[0098] The allophanate / isocyanurate composition ratio of this polyisocyanate curing agent A is 72 / 28...
Synthetic example 2
[0100] Synthesis example 2 (synthesis of curing agent B)
[0101] A polyisocyanate curing agent B was obtained in the same manner as in Synthesis Example 1, except that n-decyl alcohol was used instead of dodecyl alcohol as the monohydric alcohol.
[0102] The allophanate / isocyanurate composition ratio of this polyisocyanate curing agent B is 71 / 29, the isocyanate content is 17.0%, the viscosity (by the viscosity measured by BL type viscometer) is 26000mPa·s, unreacted h 6 The content of XDI was 0.5% by mass, and it was confirmed by NMR measurement that there was substantially no urethane bond.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com