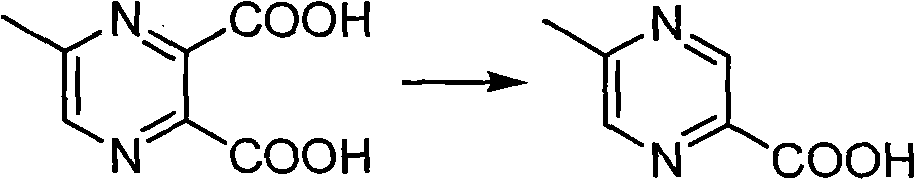
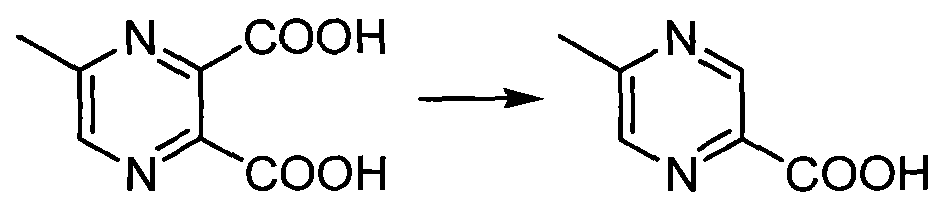Method for preparing 5-methylprazine-2-carboxylic acid
A technology of methylpyrazine and pyrazine dicarboxylic acid, applied in chemical instruments and methods, organic decomposition, organic chemistry, etc., can solve the problems of harsh conditions, unfavorable large-scale production, and high temperature, and achieves less by-products, green environmental protection Mild conditions and highly selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 80g of 5-methyl-2,3-pyrazinedicarboxylic acid to the 2L four-necked bottle And 0.96kg saturated saline (10ml / g), warming up to 110oC reflux reaction for 10h. When the reaction is carried out until the HPLC shows that the conversion rate is 91%, it is cooled to room temperature, and the filtrate is filtered with 1.6kg butanone (25mL / g), the organic phase is combined and concentrated, cooled and crystallized in an ice-water bath, and the product is obtained by suction filtration 33.5 g. HPLC purity: 98%, isomer impurity content: 1.1%, yield: 55%.
Embodiment 2
[0019] Add 36g of 5-methyl-2,3-pyrazinedicarboxylic acid to the 2L four-necked bottle And 349g saturated brine (8ml / g), warming up to 108oC reflux reaction 8h. When the reaction is carried out until the HPLC shows that the conversion rate is 92%, it is cooled to room temperature, suction filtered, and the filtrate is concentrated with 524g butanone (18mL / g), the organic phase is combined, cooled and crystallized in an ice-water bath, and the product 15.3 g. HPLC purity: 98%, isomer impurity content: 0.7%, yield: 56.2%.
Embodiment 3
[0021] Add 54g of 5-methyl-2,3-pyrazinedicarboxylic acid to the 2L four-necked bottle And 327g saturated brine (5ml / g), warming up to 105oC reflux reaction 8h. When the reaction is carried out until the HPLC shows that the conversion rate is 90%, it is cooled to room temperature, suction filtered, and the filtrate is concentrated with 655g butanone (15mL / g), the organic phase is combined, cooled and crystallized in an ice-water bath, and the product 22.5 is obtained by suction filtration. g. HPLC purity: 98%, isomer impurity content: 2.0%, yield: 55%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com