Synthetic gas preparation catalyst through reforming natural gas and carbon dioxide
A carbon dioxide and catalyst technology, which is applied in the field of catalysts for natural gas-carbon dioxide reforming to syngas, can solve problems such as difficulties in industrial scale-up, and achieve the effects of low cost, good stability and excellent performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Add 2000g spherical γ-Al with a diameter of 1mm into the container 2 o 3 Carrier, add 1020gNi(NO 3 ) 2· 6H 2 O, 30gLa(NO 3 ) 3 , 3200g of water, stirred at room temperature until dissolved. Drain the solution into the container so that it is in the Al 2 o 3 Mix evenly on the carrier and soak for 0.2 hours to make the solution completely adsorbed. The catalyst was dried at 90°C under normal pressure for 6 hours, and then calcined at 700°C for 10 hours to obtain the finished catalyst.
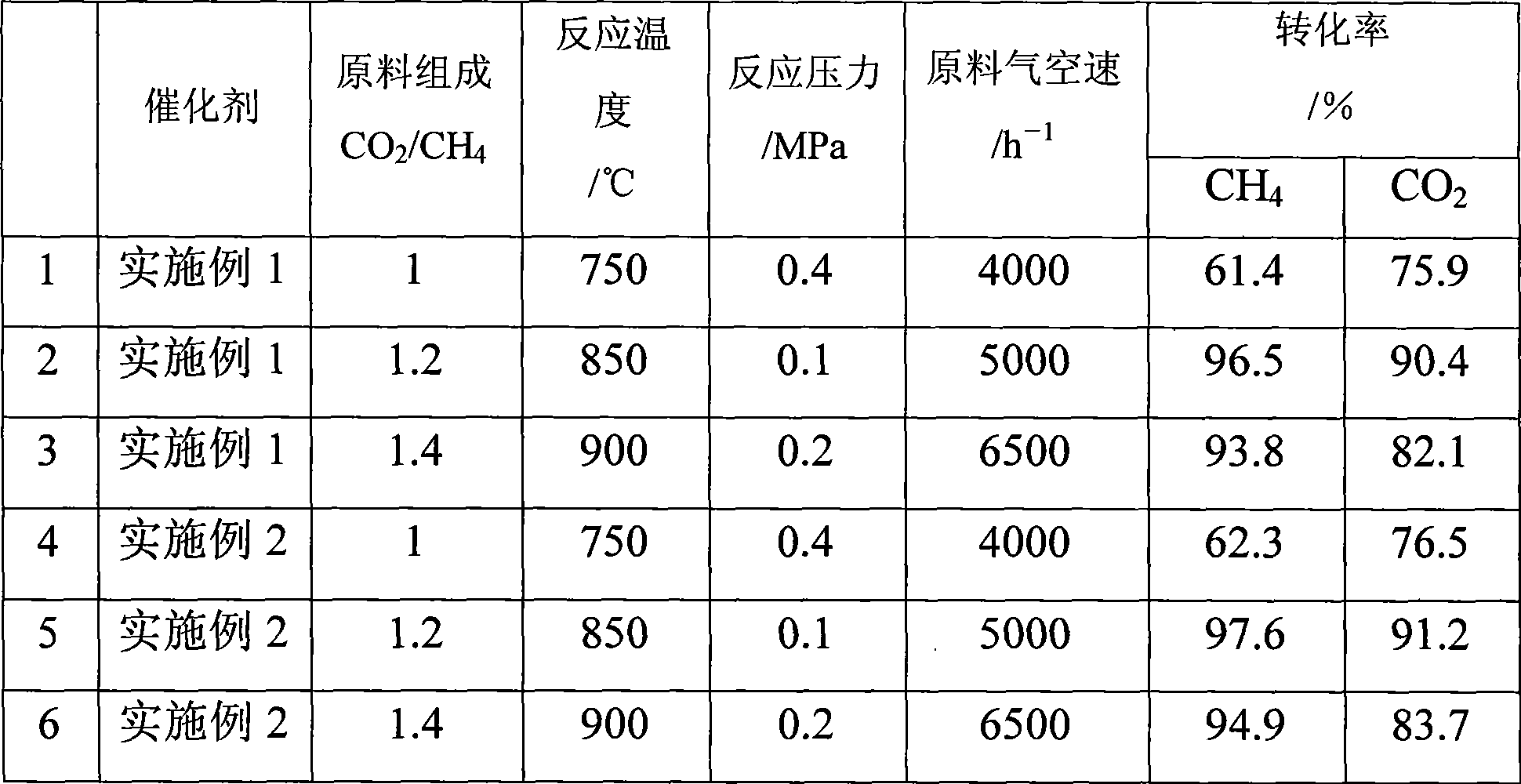
[0028] Take 300g of catalyst and place it in a reactor with a diameter of 57mm, and feed 1:1 CH 4 and CO 2 Reaction gas, its space velocity is 5000h -1 , 850 ° C, 0.1MPa pressure continuous reaction 100h. The reaction results are listed in Table 1.
[0029] Table 1 The conversion and selectivity of natural gas and carbon dioxide as a function of reaction time
[0030]
Embodiment 2
[0032] Add 2000g of spherical γ-Al with a diameter of 5mm into the container 2 o 3 Carrier, add 1900gNi(NO 3 ) 2· 6H 2 O, 30gLa(NO 3 ) 3 , 3200g of water, stirred at room temperature until dissolved. Drain the solution into the container so that it is in the Al 2 o 3 Mix evenly on the carrier and soak for 1 hour to make the solution absorb completely. The catalyst was dried at 100°C under normal pressure for 3 hours, and then calcined at 800°C for 7 hours to obtain the finished catalyst.
[0033] Take 300g of catalyst and place it in a reactor with a diameter of 57mm, and feed 1:1 CH 4 and CO 2 Reaction gas, its space velocity is 5000h -1 , 850 ° C, 0.1MPa pressure continuous reaction 100h. The reaction results are listed in Table 2.
[0034] Table 2 The conversion and selectivity of natural gas and carbon dioxide as a function of reaction time
[0035]
Embodiment 3
[0037] Add 2000g φ1.5×2~3.6mm clover-shaped molecular sieve carrier into the container, add 1020gNi(NO 3 ) 2· 6H 2 O, 30gLa(NO 3 ) 3 , 3200g of water, stirred at room temperature until dissolved. Drain the solution into a container, make it evenly mixed on the molecular sieve carrier, and soak for 0.2 hours to make the solution completely adsorbed. The catalyst was dried at 90°C for 6 hours, and then calcined at 700°C for 10 hours to obtain the finished catalyst.
[0038] Take 300g of catalyst and place it in a reactor with a diameter of 57mm, and feed 1:1 CH 4 and CO 2 Reaction gas, its space velocity is 5000h -1 , 850 ° C, 0.1MPa pressure continuous reaction 100h. The reaction results are listed in Table 4.
[0039] Table 3 The conversion and selectivity of natural gas and carbon dioxide as a function of reaction time
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com