Synthetic method of meta-hydroxyl/methoxyl polybrominated diphenyl ethers
A technology of brominated diphenyl ethers and meta-hydroxyls, which is applied in the field of synthesis of meta-hydroxyl/methoxy polybrominated diphenyl ethers, and can solve problems such as low yield, single product type, and harsh reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
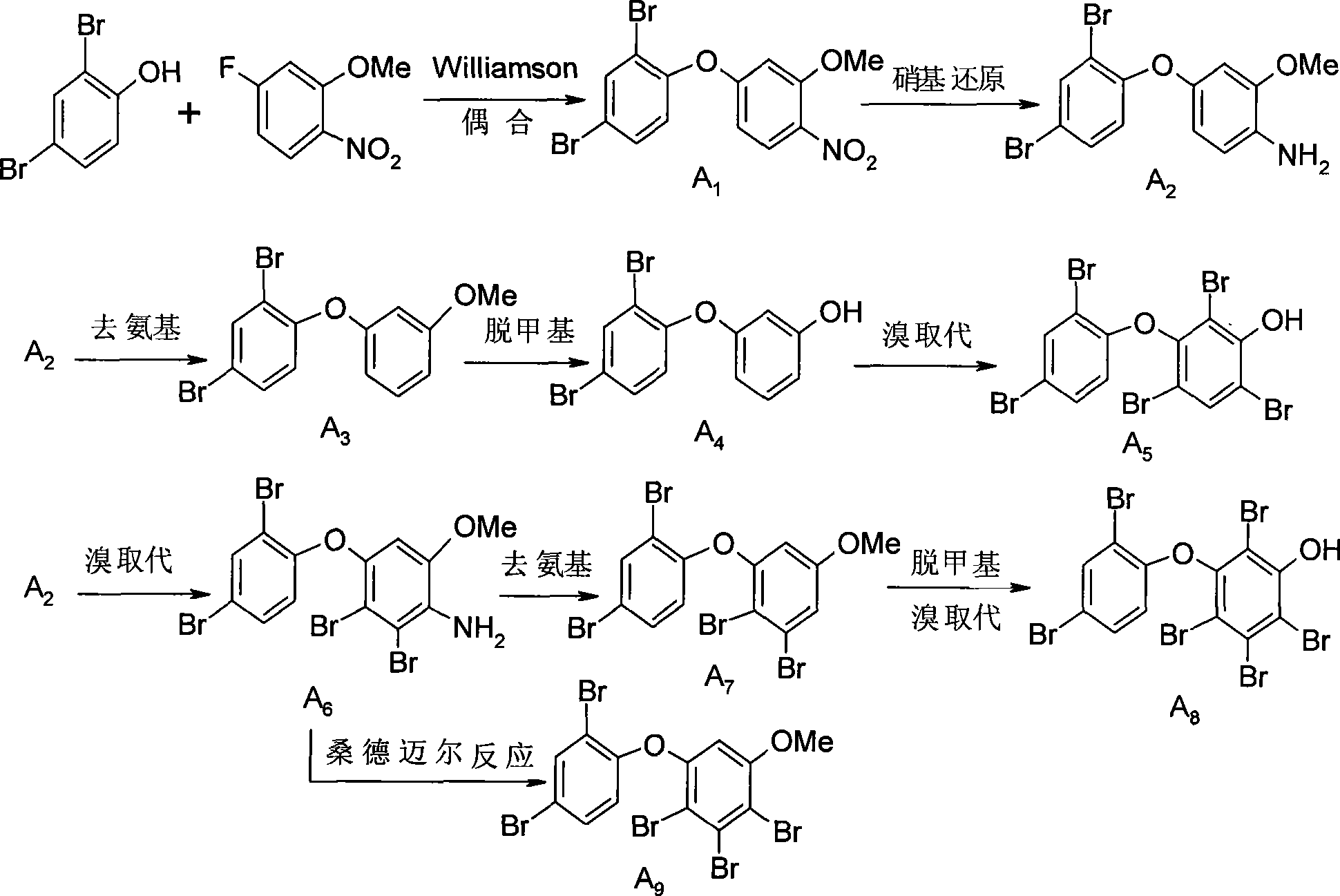
[0034] Embodiment 1: take 2,4-dibromophenol and 5-fluoro-2-nitroanisole as raw materials to synthesize meta-hydroxyl / methoxy polybrominated diphenyl ethers:
[0035] 1. coupling reaction: 2,4-dibromophenol (2.52g, 10mmol) and 5-fluoro-2-nitroanisole (2.05g, 12mmol), solvent: DMAC (10ml), add anhydrous sodium carbonate ( 10mmol), stirred and refluxed (150°C) for 1.5-2 hours. After the reaction was complete the mixture was extracted. The solvent was removed by rotary evaporation, and the crude product was separated by column chromatography to obtain pure sample A 1 , the structural formula is: Yield 95%, melting point: 139°C.
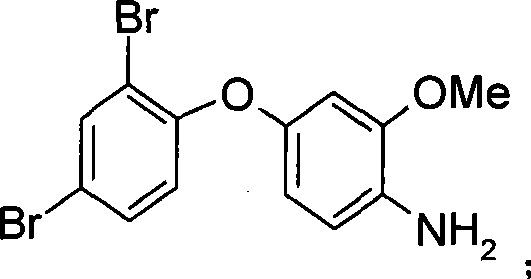
[0036] 2.A 1 (3.62g, 9mmol) reduction: the reducing agent is iron powder (9mmol), A 1 It was added to a solvent of ethanol (10ml) and acetic acid (10ml) and reacted at 80°C for about 1h. Pure sample A was obtained after extraction and column passing 2 , the structural formula is: Yield 90%, melting point: 88°C. A 2 There are two routes:
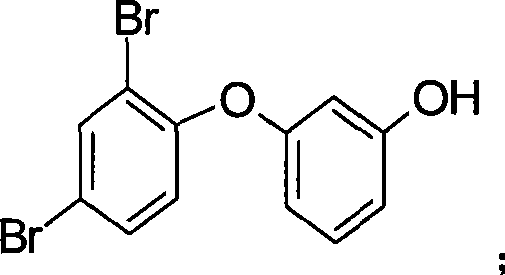
[00...
Embodiment 2
[0043] Embodiment 2: 3-aminophenol (6mmol) and 5-fluoro-2-nitroanisole reaction synthesis meta-hydroxyl / methoxy polybrominated diphenyl ethers:
[0044] 1. Coupling reaction: 3-aminophenol (6mmol) and 5-fluoro-2-nitroanisole (5mmol), solvent: DMAC (10ml), add anhydrous sodium carbonate (5mmol), stir and reflux reaction 1.5- 2 hours. After the reaction was complete the mixture was extracted. The solvent was removed by rotary evaporation, and the crude product was separated by column chromatography to obtain pure sample B 1 , the structural formula is: Yield 95%, melting point: 85°C.
[0045] 2.B 1 (1.2g, 4.5mmol) bromine substitution: with glacial acetic acid (10mmol) as solvent, Br 2 (13.5mmol) was added dropwise to B dissolved in glacial acetic acid 1 , the reaction is about 2h, the hydrogen on the ortho and para positions of the amino group is replaced by bromine, and the product is B 2 , the structural formula is: Yield 90%, melting point: 87°C.
[0046] 3. Put B...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com