Reagent for deriving small molecule aldehyde material and use thereof
A derivatization and molecular aldehyde technology, applied in the field of analytical chemistry, can solve the problems of poor selectivity of target analytes, obvious interference of impurity components, and increase the difficulty of analysis, and achieve the effects of fast derivatization, simple preparation and low price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
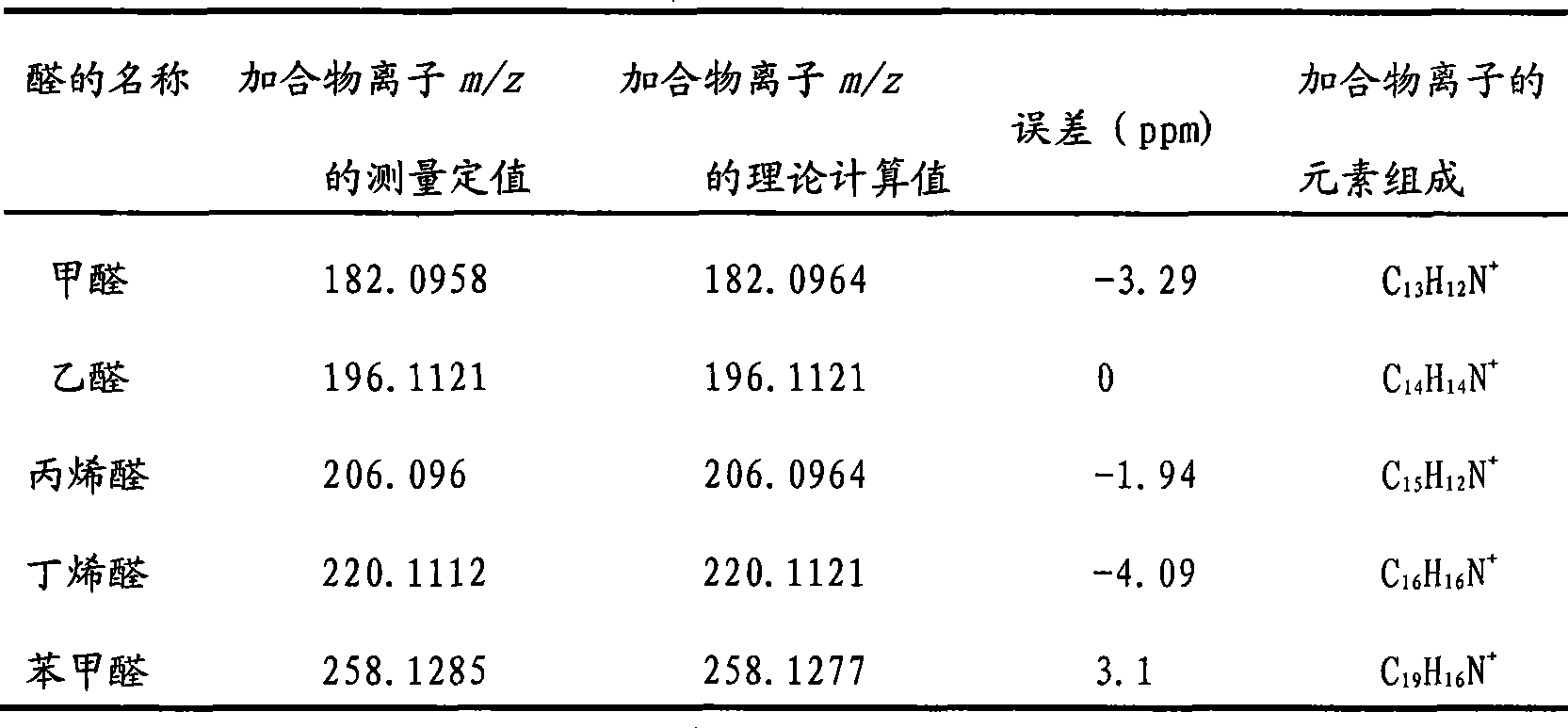
[0012] Selective analysis of small molecule aldehydes in the air: 7% diphenylamine, 35% DHB and 58% ethylene glycol were formulated as reagent (A) for derivatization of small molecule aldehydes according to the method provided by the present invention. Use a micro-syringe to absorb 2 μL of the derivatization reagent A, insert the needle of the micro-syringe into the headspace bottle that collects the air sample to be tested; carefully depress the plunger of the micro-syringe to expose the derivatization reagent to the headspace bottle, extract and derivatize After 1.5 min; suck back the droplet, drop the droplet onto the MALDI target of matrix-assisted laser desorption ionization (MALDI) Fourier transform mass spectrometry, and perform mass spectrometry analysis directly after the droplet dries to determine the aldehyde compounds (Table 1).
[0013] Table 1 adopts the derivatization reagent of the present invention to analyze the result of aldehydes in flue gas
[0014]
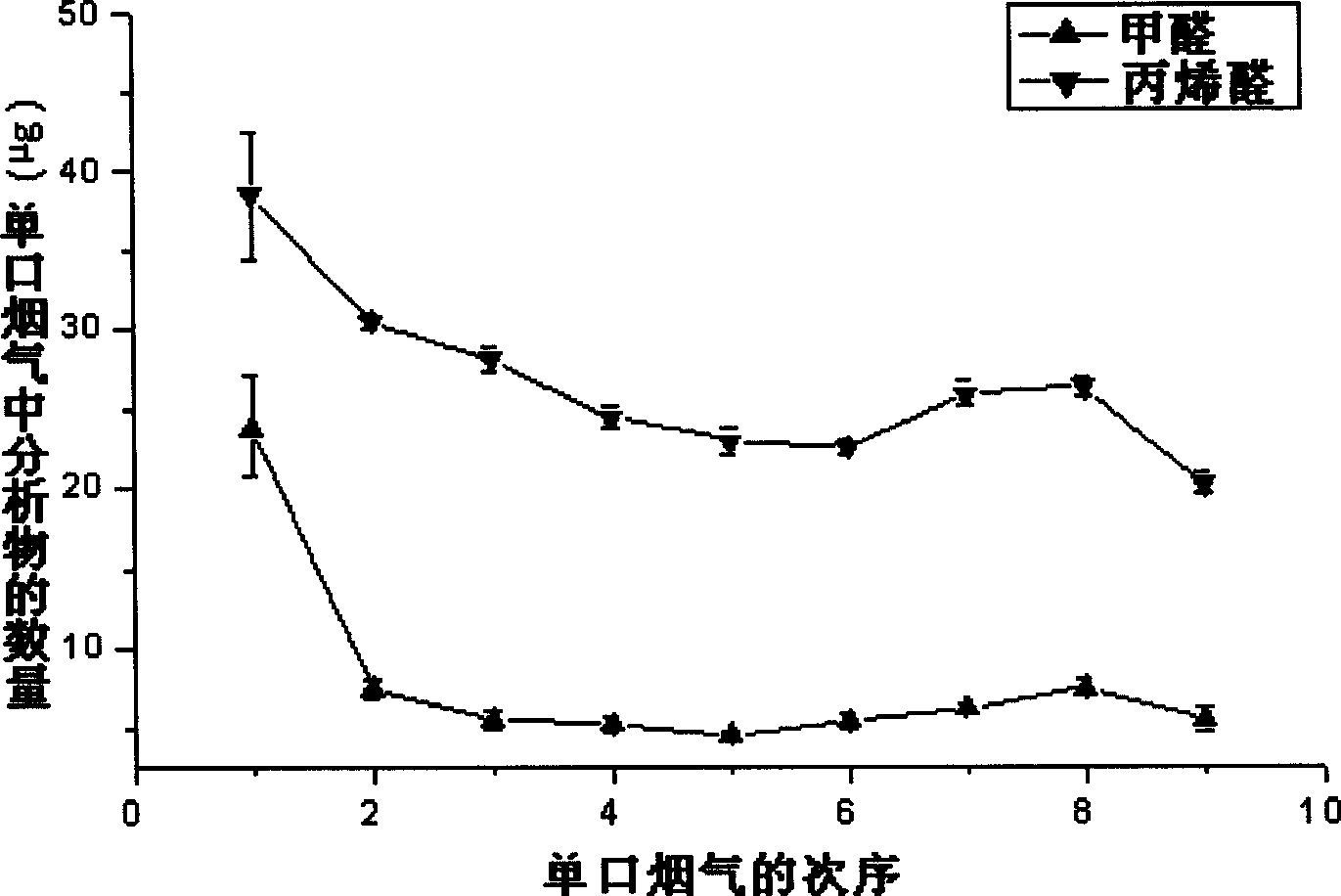
Embodiment 2
[0016] Quantitative analysis of formaldehyde and acrolein in flue gas: 5% diphenylamine, 35% DHB and 60% ethanol were formulated as reagent (B) for derivatization of small molecule aldehydes according to the method provided by the present invention. Take a certain amount of internal standard (tetrabutylammonium bromide) solution and mix with derivatization reagent to form solution C. Use a micro-syringe to absorb 1 μL of the derivatization reagent C, insert the needle of the micro-syringe into the headspace bottle where the smoke sample to be tested is collected; carefully press down the plunger of the micro-syringe to expose the derivatization reagent to the headspace bottle, extract and The derivatization time is 1min; the droplet is sucked back, and the droplet is added to the MALDI target of matrix-assisted laser desorption ionization (MALDI) Fourier transform mass spectrometry. The relative abundance of the substance was regressed to the amount of aldehyde to obtain the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com