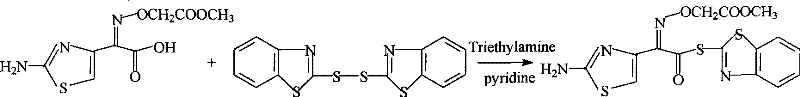
Method for preparing cefixime side chain active ester
A technology of cefixime side chain acid and active ester, applied in the preparation of 2--2-methoxycarbonylmethoxyimino-mercaptobenzothiazolyl ester, the field of cefixime side chain acid active ester, capable of Solve problems such as unfavorable actual production and increased production costs, and achieve the effects of low cost, shortened reaction cycle, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 200kg of dichloromethane into the dry reaction kettle, start stirring, add 25.9kg (100mol) of cefixime side chain acid and 40.4kg (121mol) of DM, cool down to 10°C, and add 11.1kg (115mol) of triethyl ether at one time Amine and 0.4kg (5mol) pyridine, after stirring for 30min, 22.4kg (135mol) triethyl phosphite was added dropwise within 2.5h at room temperature, then reacted at room temperature for 2.0h, cooled to -10°C and filtered with 20kg The filter cake was washed with acetonitrile and dried in vacuo to obtain 35.7 kg of orange powdery solid cefixime side chain acid active ester with a yield of 87.6% (based on cefixime side chain acid) and a content greater than 98.5%.
Embodiment 2
[0018] Add 380kg of dichloromethane into the dry reaction kettle, start stirring, add 25.9kg (100mol) of cefixime side chain acid and 46.6kg (140mol) of DM, cool down to -5°C, and add 24.1kg (130mol) of three n-Butylamine, after stirring for 30min, add 24.9kg (150mol) triethyl phosphite dropwise within 1.0h at room temperature, then react at room temperature for 4.0h, cool down to -10°C, filter by rejection, and wash with 20kg of dichloromethane The filter cake was vacuum-dried to obtain 11.3 kg of orange powdery solid cefixime side-chain acid active ester, with a yield of 27.6% (calculated as cefixime side-chain acid), and a content greater than 98.5%.
Embodiment 3
[0020] Add 130kg of toluene to the dry reaction kettle, start stirring, add 25.9kg (100mol) of cefixime side chain acid and 33.3kg (100mol) of DM, control the temperature at 30°C, and add 10.1kg (100mol) of triethylamine at one time and 1.0kg (10mol) lutidine, after stirring for 30min, add 18.3kg (110mol) triethyl phosphite dropwise within 4.0h at 30°C, then react at 30°C for 2.0h, and cool down to -10°C , filter by rejection, and wash the filter cake with 20kg toluene, get 32.0kg orange-yellow powdery solid cefixime side chain acid active ester after vacuum drying, yield 78.4% (in terms of cefixime side chain acid), and content is greater than 98.5% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com