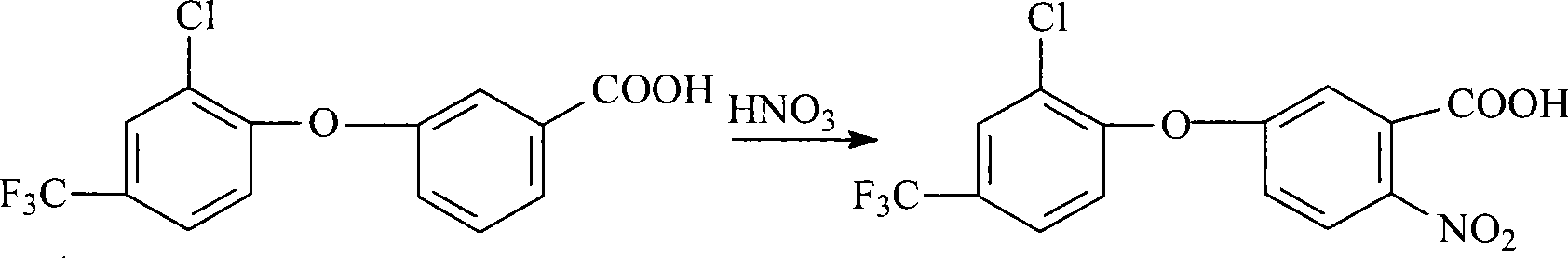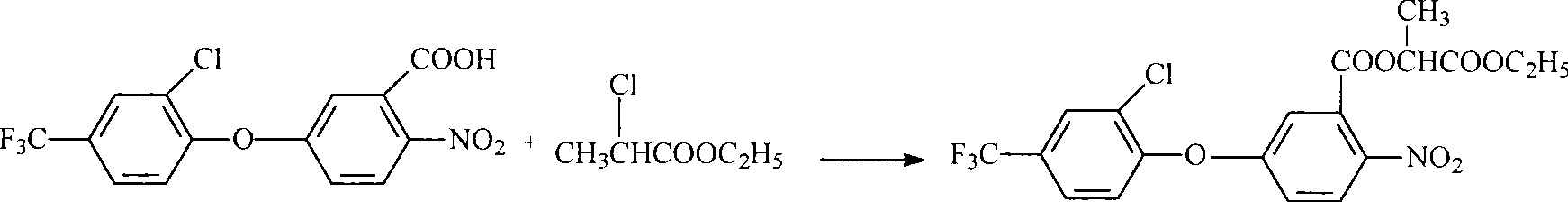Loctofen preparation method
A technology for the preparation of lactoferin and lactoferin, which is applied in the field of the preparation of lactoferin-ethyl raw medicine, to achieve the effects of high content of the original medicine, reduction of by-product formation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
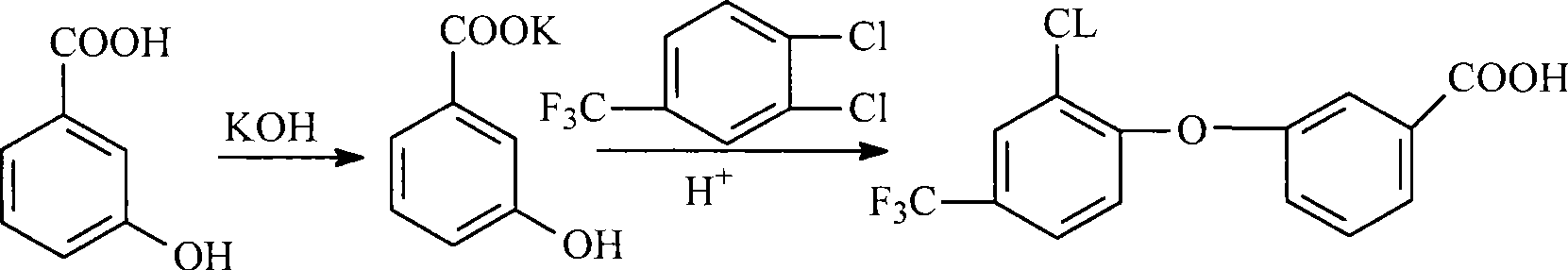
[0037] 1) Preparation of 3-[2-chloro-4-(trifluoromethyl)phenoxy]benzoic acid
[0038] In a 100L reactor, add 38.60Kg dimethyl sulfoxide (99%, 490mol) and put it into the reactor after metering, and add 10.10Kg potassium hydroxide (93%, 168mol) and 10.45Kg m-hydroxybenzene while stirring Formic acid (98%, 74.2 mol) was heated to 110° C. for a salt-forming reaction for 3 hours, and the water and part of the solvent generated by the reaction were removed under reduced pressure. Then add 3.45Kg anhydrous potassium carbonate (98%, 24.5mol) and 15.28Kg 3.4-dichlorobenzotrifluoride (98.5%, 70mol) in still, continue to heat up to 152-158 ℃, carry out condensation reaction, condensation reaction 20 -22 hours, the solvent dimethyl sulfoxide was removed. Add water to dissolve the remaining solids in the kettle, add concentrated hydrochloric acid dropwise to the solution for acidification to make PH=1-2, after the acidification is completed, carry out centrifugation, dry the wet product ...
Embodiment 2
[0045] 1) Preparation of 3-[2-chloro-4-(trifluoromethyl)phenoxy]benzoic acid
[0046] In a 100L reactor, add 38.60Kg dimethyl sulfoxide (99%, 490mol) and put it into the reactor after metering, and add 10.10Kg potassium hydroxide (93%, 168mol) and 10.45Kg m-hydroxybenzene while stirring Formic acid (98%, 74.2 mol) was heated to 110° C. for a salt-forming reaction for 3 hours, and the water and part of the solvent generated by the reaction were removed under reduced pressure. Then add 3.45Kg anhydrous potassium carbonate (98%, 24.5mol) and 15.28Kg 3.4-dichlorobenzotrifluoride (98.5%, 70mol) in still, continue to heat up to 152-158 ℃, carry out condensation reaction, condensation reaction 20 -22 hours, the solvent dimethyl sulfoxide was removed. Add water to dissolve the remaining solids in the tank, add concentrated hydrochloric acid dropwise to the solution for acidification to make PH = 1-2, after the acidification is completed, perform centrifugation, and dry the cake to ob...
Embodiment 3
[0053] Repeat the same steps as described in Example 1, but from step 3): the preparation of lactofen: in a 100L reactor, add 73.25Kg ethyl 2-chloropropionate (99%, 530mol), 19.86Kg 5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid (91.0%, 50mol) was added under stirring, and the temperature was raised to 80-85°C to obtain solution A. In a 150L reactor, add 43.95Kg ethyl 2-chloropropionate (99%, 320mol) and 9.15Kg potassium carbonate (99%, 65mol), stir and heat up at 80-85°C to obtain solution B, and at 80-85°C, dissolve A Add the solution dropwise into solution B. After the dropwise addition, heat up to 90-95°C and add 0.2Kg tetrabutylammonium bromide, then keep it warm at 90-95°C for 6-8 hours, measure the reaction end point by HPLC, filter, and add water to the filtrate Wash with water until neutral, remove the water layer, remove excess ethyl 2-chloropropionate from the oil layer to obtain 24.4Kg of lactofen-alifop, with a content of 87.3%, and a yield of 92.3%, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com