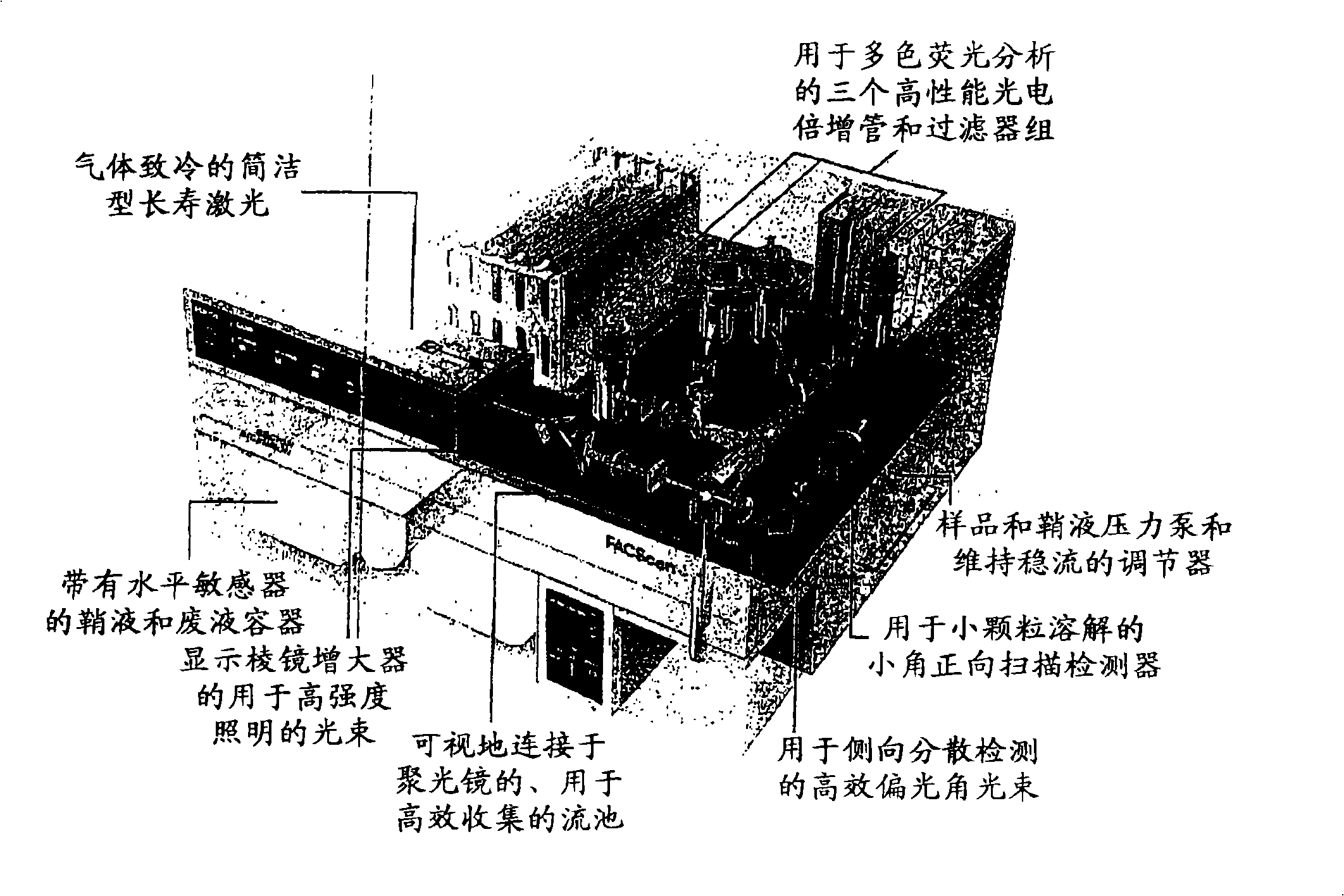
Human papilloma virus (HPV) detection using nucleic acid probes, microbeads and fluorescent-activated cell sorter (FACS)
A probe, nucleic acid capture technology, used in the determination/inspection of microorganisms, particle and sedimentation analysis, measuring devices, etc., can solve the problems of indistinguishable, unsatisfactory cervical cancer risk, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0274] Example 1: HPV Diagnosis - DNA Isolation and Amplification
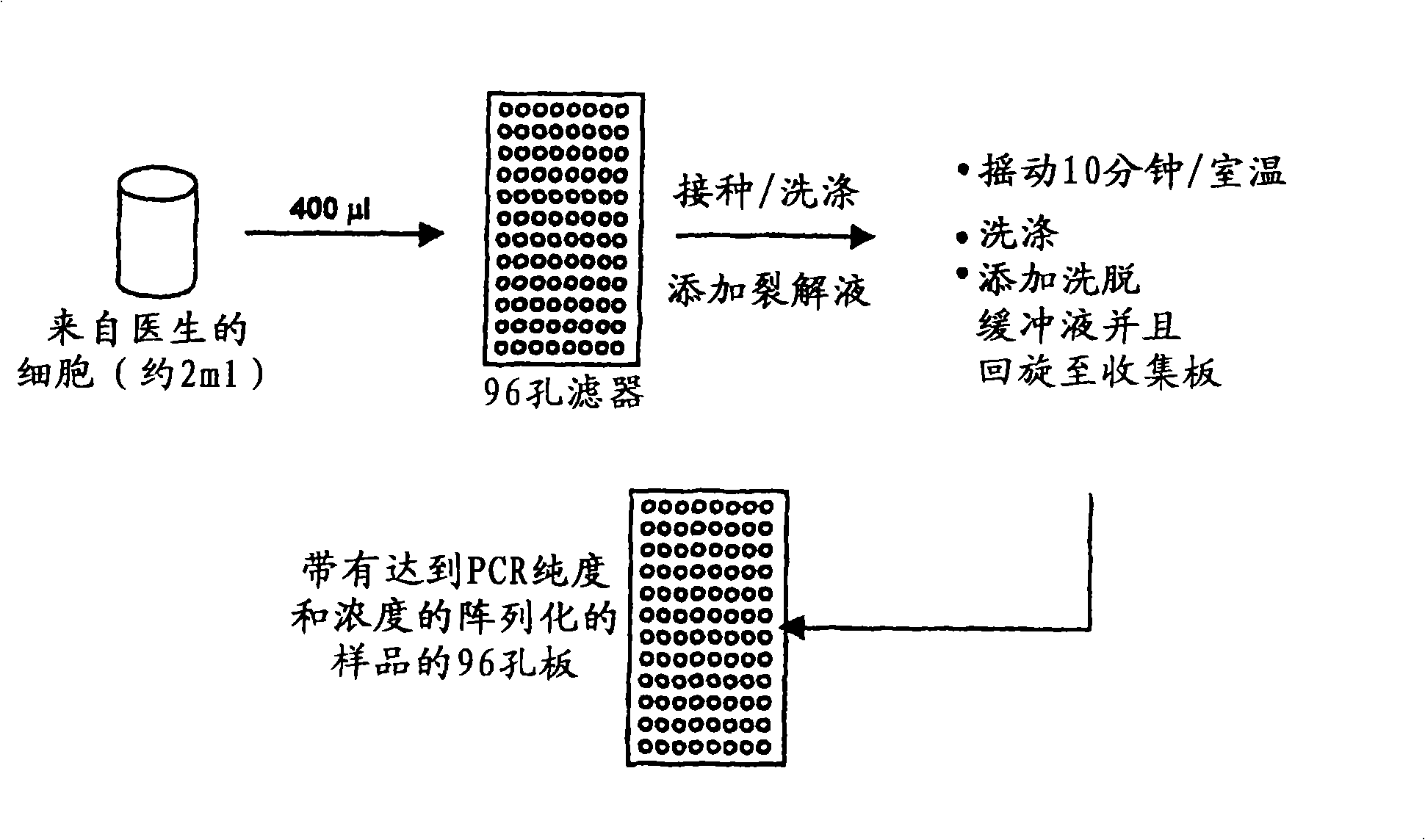
[0275] exist figure 2 An overview of DNA extraction methods used to isolate DNA in HPV diagnostic methods is shown in .
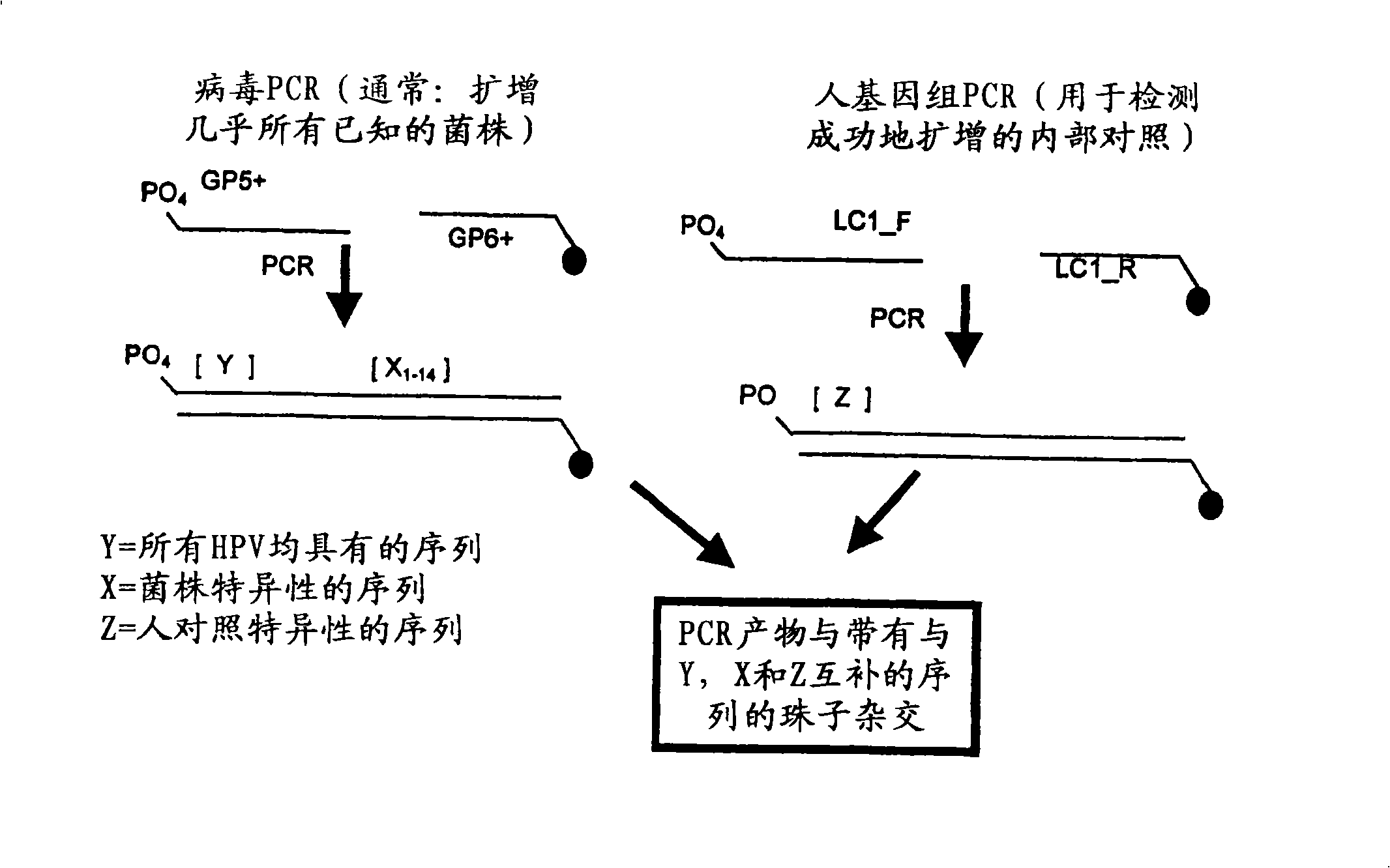
[0276] Such as image 3 DNA samples were amplified using PCR as indicated. Primers GP5+ and GP6+ were used to generate amplicons of any HPV strain present in the DNA sample. Primer GP6+ contains a fluorescent label, more specifically Cy5, to allow for later visualization of the binding of the amplicon to the binding reagent. The resulting viral amplicon contains a conserved region (Y), which is conserved among all HPV strains examined, and a region X that is variable (i.e., strain-specific) between HPV strains n , where n represents the variable region associated with each HPV strain. The binding partners immobilized on the beads will specifically bind the HPV strain-specific genome.
[0277] In addition, amplicons from human subject genomic DNA were also generated using LC1_F and LC...
Embodiment 2
[0278] Example 2: HPV Diagnosis - Multiplex Detection
[0279] The amplicons generated in Example 1 were hybridized to an array of binding reagents each carrying a protein from each HPV strain c, 11, 16, 18, 31, 33, 35, 42, 45, 51, 52 , 56, 58, 59, 67 and 68 (X 1 to X 16 ) produced a polynucleotide complementary to the variable region of a putative viral amplicon. For the nucleotide sequence of the capture nucleic acid immobilized on the beads see Figure 9 . Furthermore, the array comprises binding reagents comprising polynucleotides complementary to the conserved region (Y) of the HPV viral amplicon. Finally, a binding reagent comprising a polynucleotide complementary to the human control amplicon sequence is included. Capture nucleic acid can be DNA or RNA. If RNA is used, reverse transcriptase may be required to generate RNA from DNA amplicons.
[0280] Each binding reagent in the array comprises microspheres or beads with different sizes and different fluorescent (T...
Embodiment 3
[0284] Example 3: Comparison of multiple detection methods and traditional HPV diagnosis
[0285] Table 5 below provides an overview comparing the multiplex HPV detection method of the present invention with existing histological methods for HPV diagnosis.
[0286] Table 5 Comparison of HPV diagnostic methods
[0287] HPV diagnostic methods
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com