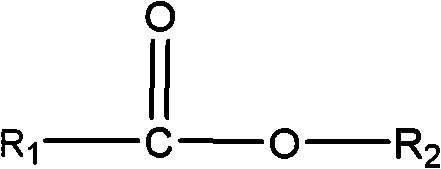
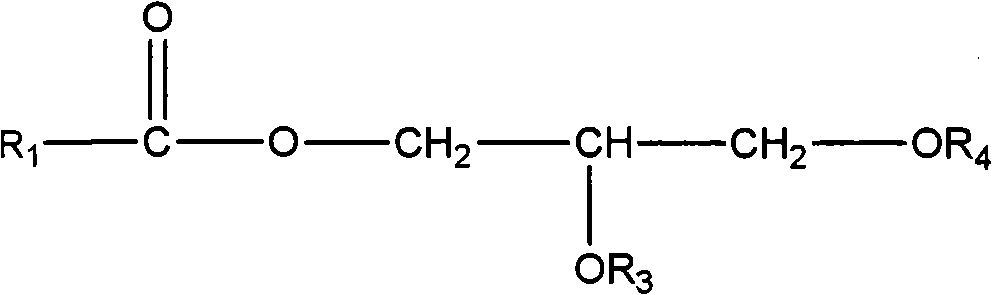
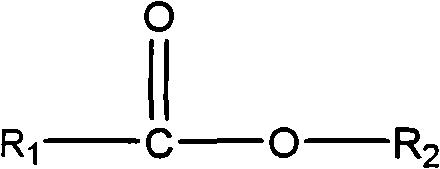
Production of peracids using an enzyme having perhydrolysis activity
A technology for the production of peroxycarboxylic acid and peracid, applied in the direction of fermentation, etc., can solve the problems of unstable peracid, insufficient use, reduction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0204] Bacillus subtilis ATCC 31954 TM Growth and preparation of cell extracts
[0205] After the dried culture was suspended in 5 mL of nutrient medium (Difco; 0003-01-6) and incubated at 30°C for 3 days, Bacillus subtilis (ATCC 31954 TM ) cultures were rejuvenated. After 3 days of incubation, aliquots of the culture were streaked on Trypticase Soy Agar plates (Becton, Dickinson and Company; Franklin Lakes, NJ) and incubated at 35°C for 24 hours. A few single colonies were scraped onto a 1 microliter inoculation loop (Becton Dickinson; Cat #220215) and transferred into 50 mL of Lactobacillus MRS Broth (Hardy Diagnostics, Santa Maria, CA; Cat #C5931 ). The culture was then grown for 12 hours at 30°C and a 200-rpm agitation rate. After 12 hours of growth, 2 mL of the culture was transferred to an unbaffled 500-mL shake flask containing 100 mL of MRS broth for 12-14 hours of growth at 30°C with a 200-rpm agitation rate. Cells were then harvested by centrifugation at 15...
Embodiment 2
[0208] Bacillus subtilis ATCC 31954 TM semi-purified cell extract Determination of perhydrolytic activity
[0209] 1.0-mL aliquot of Bacillus subtilis (ATCC 31954 TM ) cell extract (10 mg total protein / mL, prepared as described in Example 1) was diluted with an equal volume of 50 mM phosphate buffer (pH 7.0) and passed through a 100,000 Molecular Weight Cutoff (MWCO) Centricon unit membrane (Millipore Corp, Bedford, MA) for filtering. The resulting filtrate (semi-purified cell extract) contained 1.5 mg total protein / mL, and assay of this filtrate indicated no measurable catalytic enzyme activity.
[0210] A 1-mL reaction containing triacetin (250 mM), hydrogen peroxide (2.5 M) and 0.100 mL of semi-purified cell extract (0.15 mg extracted total protein) in 50 mM phosphate buffer (pH 6.5) The mixture was mixed at 25°C. A control reaction was performed by replacing the semi-purified cell extract with 50 mM phosphate buffer (pH 6.5) to determine the rate of passage o...
Embodiment 3
[0224] Semi-purified enzyme from Bacillus subtilis ATCC cell extract perhydrolytic activity
[0225] Bacillus subtilis ATCC 31954 TM Growth and extract preparation were performed as described in Example 1, except that the crude extract was not centrifuged. The crude extract was subjected to fractional distillation with cold n-propanol (-20°C). The flask containing the cell-free extract was agitated in an ice bath for 15 minutes before adding dropwise (to prevent freezing of the extract) n-propanol (-20°C) to a concentration of 40% (v / v). The resulting extract / propanol mixture was agitated in an ice bath for 30 minutes, then centrifuged at 12,000 xg for 10 minutes at 5°C, and the supernatant was returned to the flask and placed in an ice bath. Additional n-propanol (-20°C) was slowly added to the supernatant with agitation to a concentration of 60% (v / v), and the resulting mixture was agitated in an ice bath for 30 minutes and centrifuged as before. The pellet from this ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com