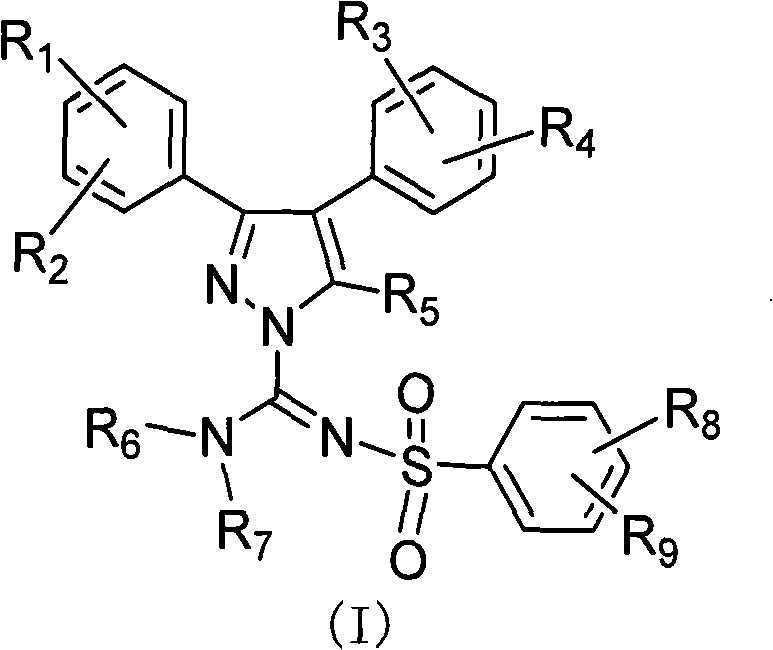
Diaryl substituted pyrazole derivative, preparation method and application thereof
A methyl and alkyl technology, applied in the field of novel diaryl substituted pyrazole derivatives, can solve the problems of unsatisfactory affinity and low CB1 receptor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] In the preparation method of the present invention, each reaction is usually carried out in an inert solvent at room temperature to solvent reflux temperature (such as 0°C-80°C, preferably 0°C-50°C). The reaction time is usually 0.1-60 hours, preferably 0.5-48 hours.
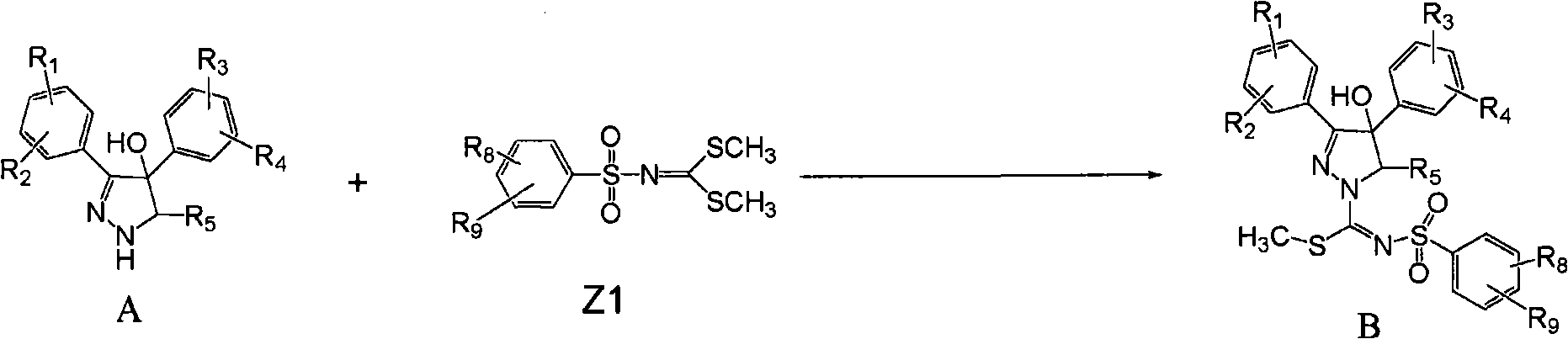
[0055] In a preferred example, the compound of formula (I) of the present invention can be prepared according to the following process:
[0056] Reaction scheme I
[0057]
[0058] Step 1. Compound 1 [J.Med.Chem.2004,47,627] and compound 7 [Chem.Ber.1966,99,2885;.J.Med.Chem.2004,47,627] in the presence of a base, Compound 2 can be obtained by reacting in a suitable solvent.
[0059] The base suitable for the above reaction is an organic base and an inorganic base, and the most suitable base is triethylamine, pyridine, dimethylaminopyridine; the solvent suitable for the above reaction is acetonitrile, DMF, pyridine, methylene chloride, DMSO, tetrahydrofuran, benzene , Toluene.
[0060] The reaction ...
Embodiment 1
[0126] Example 1: 3-(4-chlorophenyl)-N-(4-chlorophenylsulfonyl)-4-hydroxy-4-phenyl-4,5-dihydro-1H-pyrazole-1-carbonyl Methyl iminothioate (2a)
[0127] Compound 1 (12.75g, 46.8mmol) and compound 7a (17.73g, 60.0mmol) were dissolved in a mixed solution of acetonitrile (200ml) and triethylamine (20ml), and the mixture was heated to reflux until compound 1 was completely reacted. Concentrate the reaction mixture to dryness, add dichloromethane, wash twice with water, dry over sodium sulfate, and concentrate the residue. %). 1 NMR (CDCl 3)δ: 2.31(s, 3H), 4.62(d, 1H, J=12.18Hz), 4.83(d, 1H, J=12.18Hz), 7.20-7.40(m, 9H), 7.66(dt, 2H, J = 6.97 Hz), 7.82 (dt, 2H, J = 6.78 Hz).
Embodiment 2
[0128] Example 2: N 1 -Methyl-N 2 -(4-chlorophenylsulfonyl)-3-(4-chlorophenyl)-4-hydroxy-4-phenyl-4,5-dihydro-1H-pyrazole-1-carbonylamidine (3a)
[0129] Compound 2a (1.2g, 2.3mmol) was dissolved in a mixed solution of methanol (30ml) and dichloromethane (30ml), and methylamino alcohol solution (18ml) (or excess other amines) was added at room temperature. Stir until compound 2a reacts completely. The reaction mixture was concentrated to dryness, and the residue was directly subjected to column chromatography (developing solvent: petroleum ether: ethyl acetate = 5:1) to obtain compound 3a (0.6 g, 51.7%). 1 NMR (DMSO) δ: 2.95 (s, 3H), 4.13 (d, 1H, J = 12.18Hz), 4.30 (d, 1H, J = 12.18Hz), 7.27-7.57 (m, 9H), 7.65-7.68 ( m, 1H), 7.76-7.85 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com