Vaccine for preventing and/or treating allergic asthma
A technology for allergic asthma and vaccines, applied in allergic diseases, respiratory diseases, allergen antigen components, etc., can solve problems such as Candida albicans infection, hypertension, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
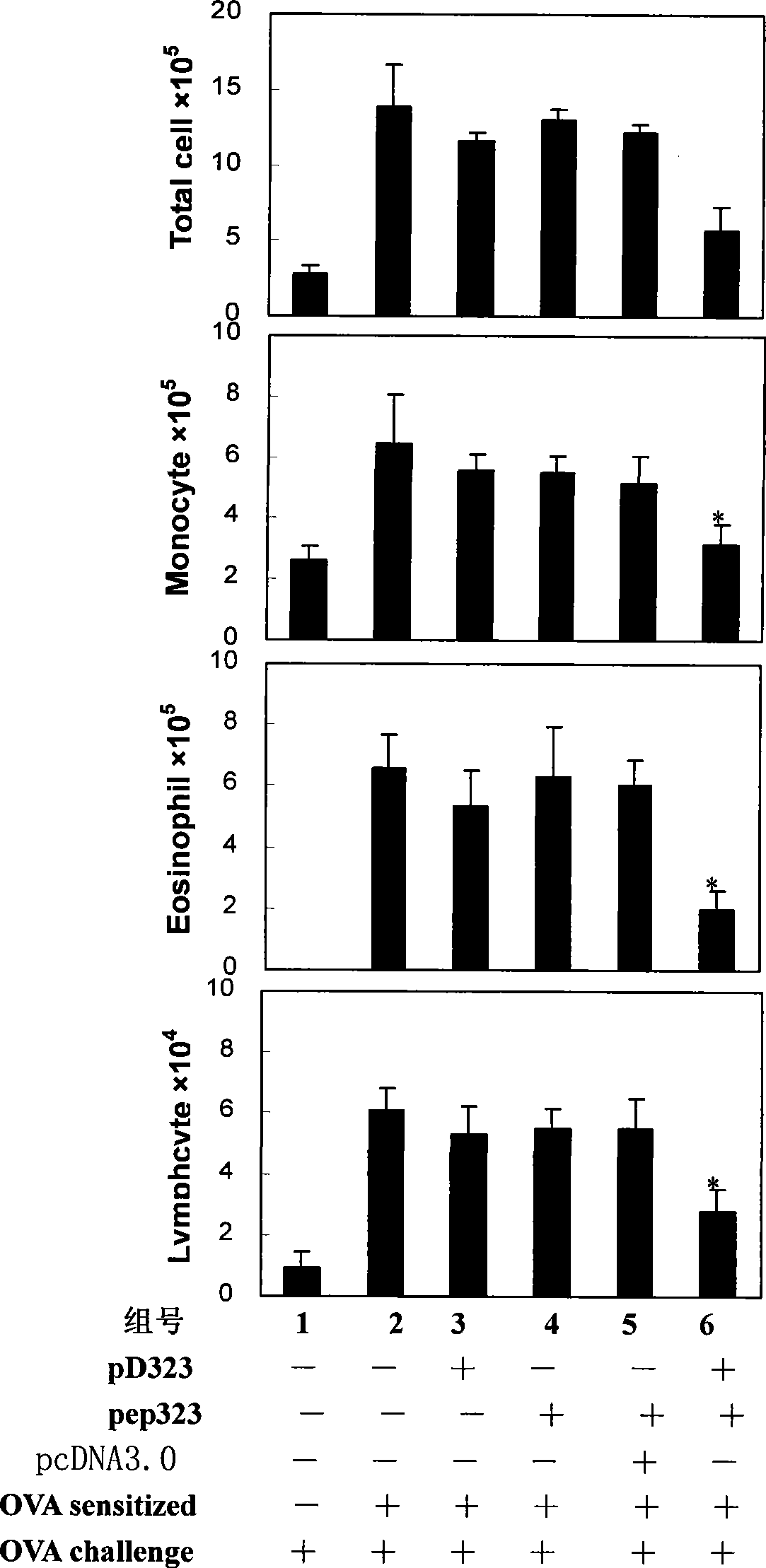
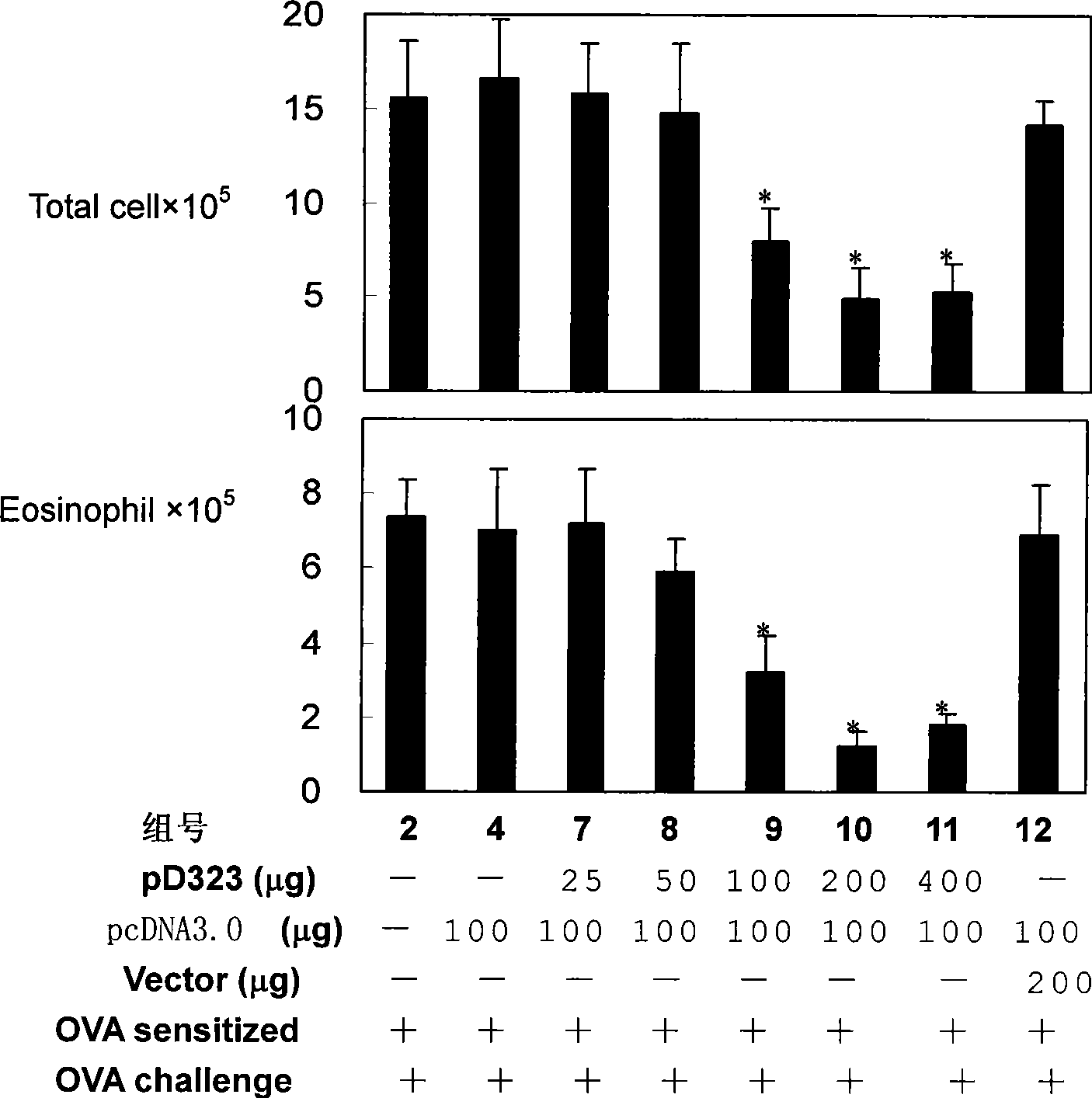
[0046] Embodiment 1, pep323 and pD323 joint immunosuppressive asthma
[0047] experimental method
[0048] Experimental animals: female BALB / c mice (8-10 weeks old) were purchased from the Institute of Experimental Animals, Chinese Academy of Medical Sciences. DO11.10 mice (8-10 weeks old) were purchased from Shanghai Slack Experimental Animal Co., Ltd. (SLAC).
[0049] Neutralizing antibodies: various neutralizing antibodies used in the experiment, including anti-CD25, anti-CD4, anti-IL-10, anti-IFN-γ, anti-TGF-β and anti-IL-4, anti-IL -2 were obtained from hybridoma cells purchased from ATCC (Manassas, VA, USA).
[0050] 1. Experimental method
[0051] 1. Asthma model induction: OVA (Sigma, USA; 1 mg / ml dissolved in PBS) was mixed with an equal volume of 10% aluminum salt (Sigma, USA) as an allergic antigen, and oscillated for 2 hours at room temperature. After centrifugation at 750×g for 5 min, the supernatant was discarded and the OVA / aluminum complex was resuspended w...
Embodiment 2
[0092] Example 2, Der p1 protein and pVAX-Der p1 combined immunosuppressive asthma
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com