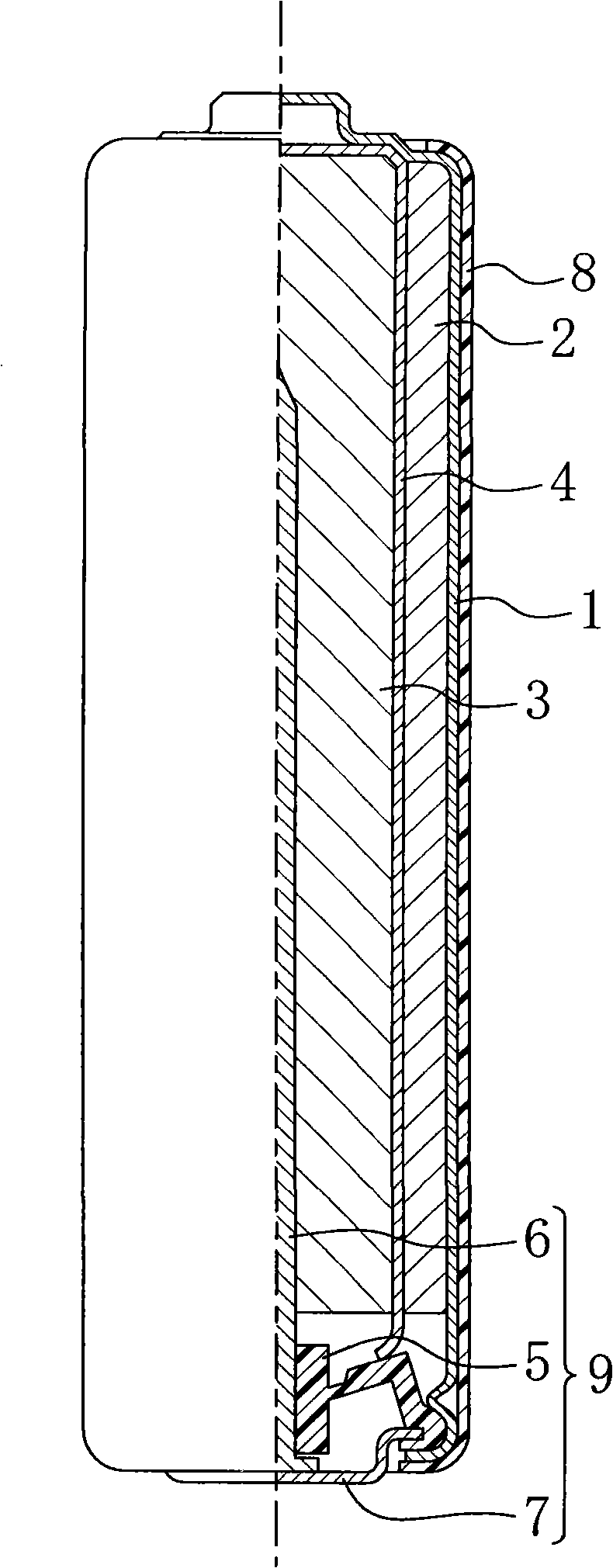
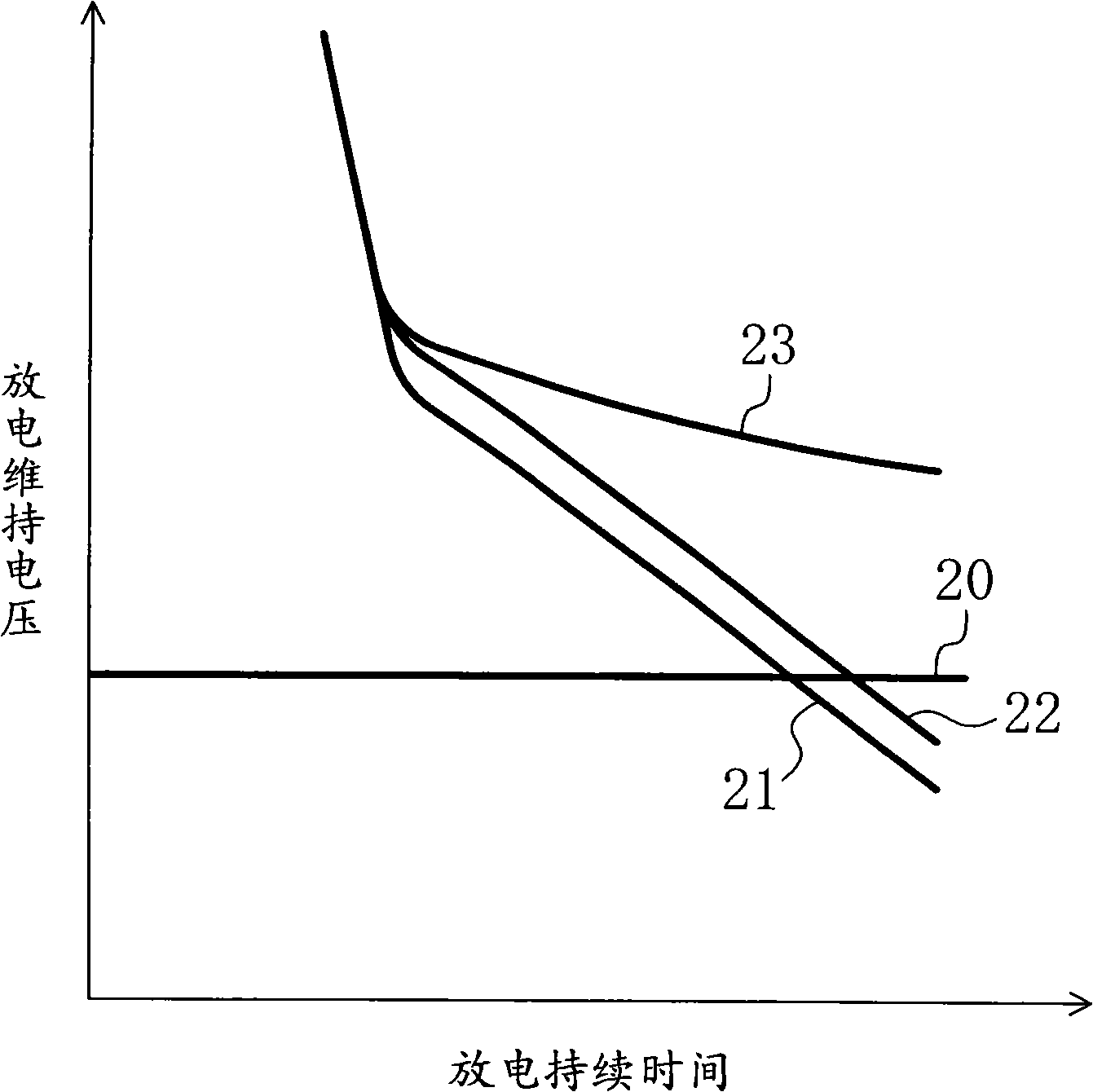
Alkaline cell
A dry battery, alkaline technology, applied in the field of alkaline dry batteries, can solve the problems of decreased discharge capacity, reduced electrode reaction speed, low utilization rate, etc., to achieve the effect of inhibiting polarization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
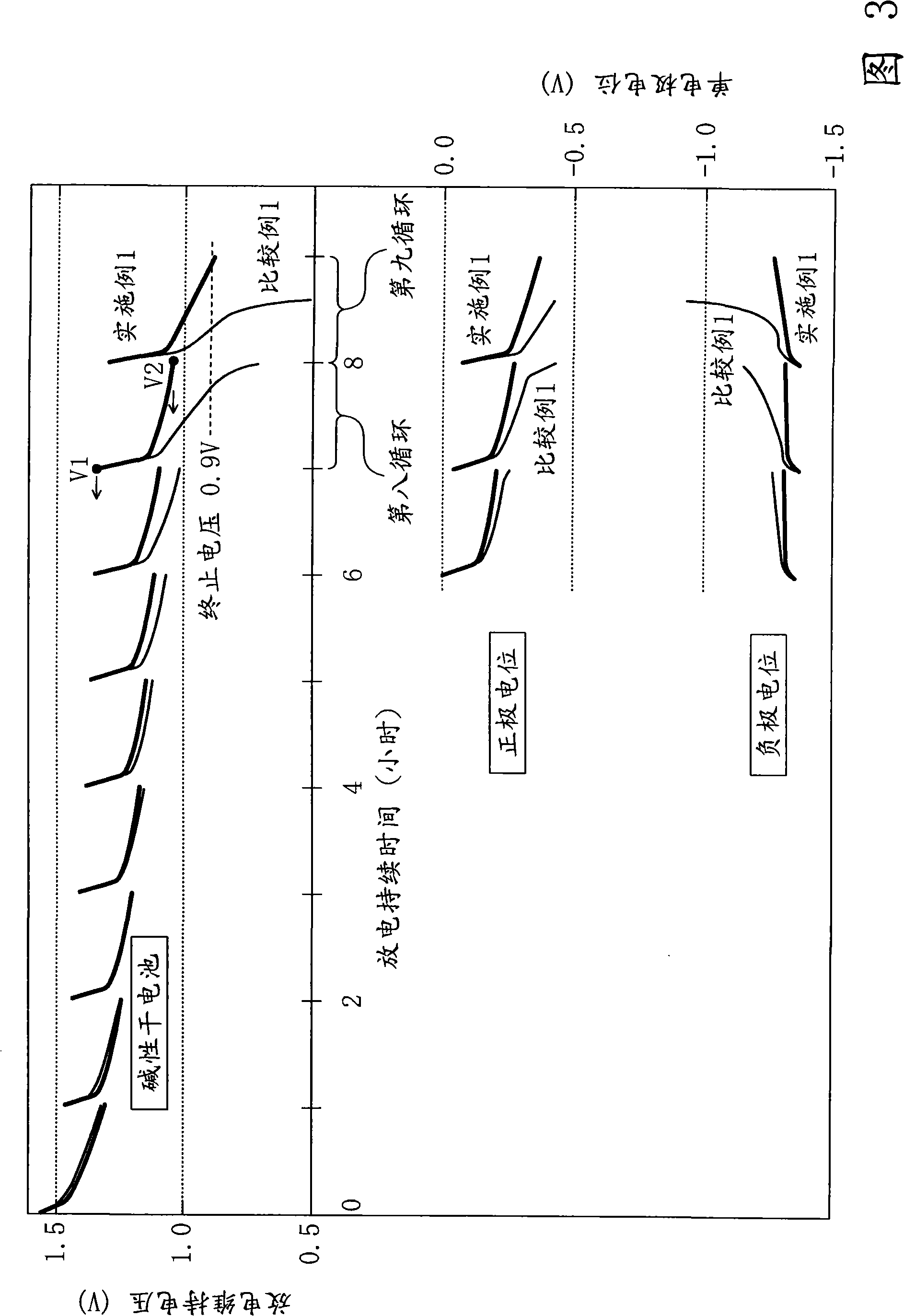
Embodiment 1 and comparative example 1
[0134] Compound 1 represented by the following (Chemical Formula 4) was synthesized by an esterification reaction between alkyl polyoxyethylene alcohol and phosphoric acid.
[0135] [chemical formula 4]
[0136]
[0137] In addition, (Chemical Formula 4) is an organic compound represented by the above-mentioned (Chemical Formula 1).
[0138] In Example 1, first, in the preparation of the above-mentioned alkaline electrolyte solution, 0.5% by weight of compound 1 was added to the alkaline electrolyte solution, and it was dissolved after sufficiently stirring.
[0139] Next, using the alkaline electrolyte and according to the prescribed conditions shown in (Table 1), AAA alkaline dry batteries were produced in accordance with the steps "2" to "4".
[0140] In addition, in the production of the above-mentioned "1" alkaline electrolyte, except that an alkaline electrolyte without any addition was prepared, the same single 3-shaped alkaline dry battery as in Example 1 was prod...
Embodiment 2~8 and comparative example 2~3
[0153] Through the esterification reaction between alkyl alcohol, polyethylene glycol and phosphoric acid, polyoxyethylene alkyl ether phosphate as shown in the following general formula (chemical formula 5) is obtained.
[0154] [chemical formula 5]
[0155]
[0156] In (chemical formula 5), R is -CH 2 CH 2 -or-CH(CH 3 )CH 2 -.
[0157] In addition, (Chemical Formula 5) is an organic compound represented by the above-mentioned (Chemical Formula 1) or an alkali metal salt thereof.
[0158]In addition, by changing the degree of polymerization of ethylene glycol in polyethylene glycol, the number of carbon atoms of the alkyl group in the alkyl alcohol, and the type of salt that neutralizes the phosphoric acid group, as shown in Table 2, as the general formula (chemical formula 5 ) in the structural parameters m, n, X and Y are combined to produce compounds 2-10.
[0159] Incidentally, Compound 1 used in Example 1 is a compound when m=4, n=1, and X, Y="H (hydrogen atom...
Embodiment 9~16 and comparative example 4~6
[0170] Phosphoric acid ester represented by the following general formula (chemical formula 6) is obtained by the esterification reaction between alkyl alcohol and phosphoric acid.
[0171] [chemical formula 6]
[0172]
[0173] In addition, (Chemical formula 6) is an organic compound represented by the above-mentioned (Chemical formula 2) or an alkali metal salt of the organic compound.
[0174] In addition, by changing the number of carbon atoms of the alkyl group in the alkanol and the type of salt that neutralizes the phosphate group, as shown in Table 3, m, n, m+ as structural parameters in the general formula (chemical formula 6) Compounds 11 to 21 were prepared by variously changing n, X, and Y.
[0175] In addition, in the preparation of the above-mentioned "1" alkaline electrolyte, 0.5% by weight of compounds 11 to 21 was added to the alkaline electrolyte, and after fully stirring and dissolving, a single A 3-shaped alkaline dry battery, and a 250 mA intermittent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com