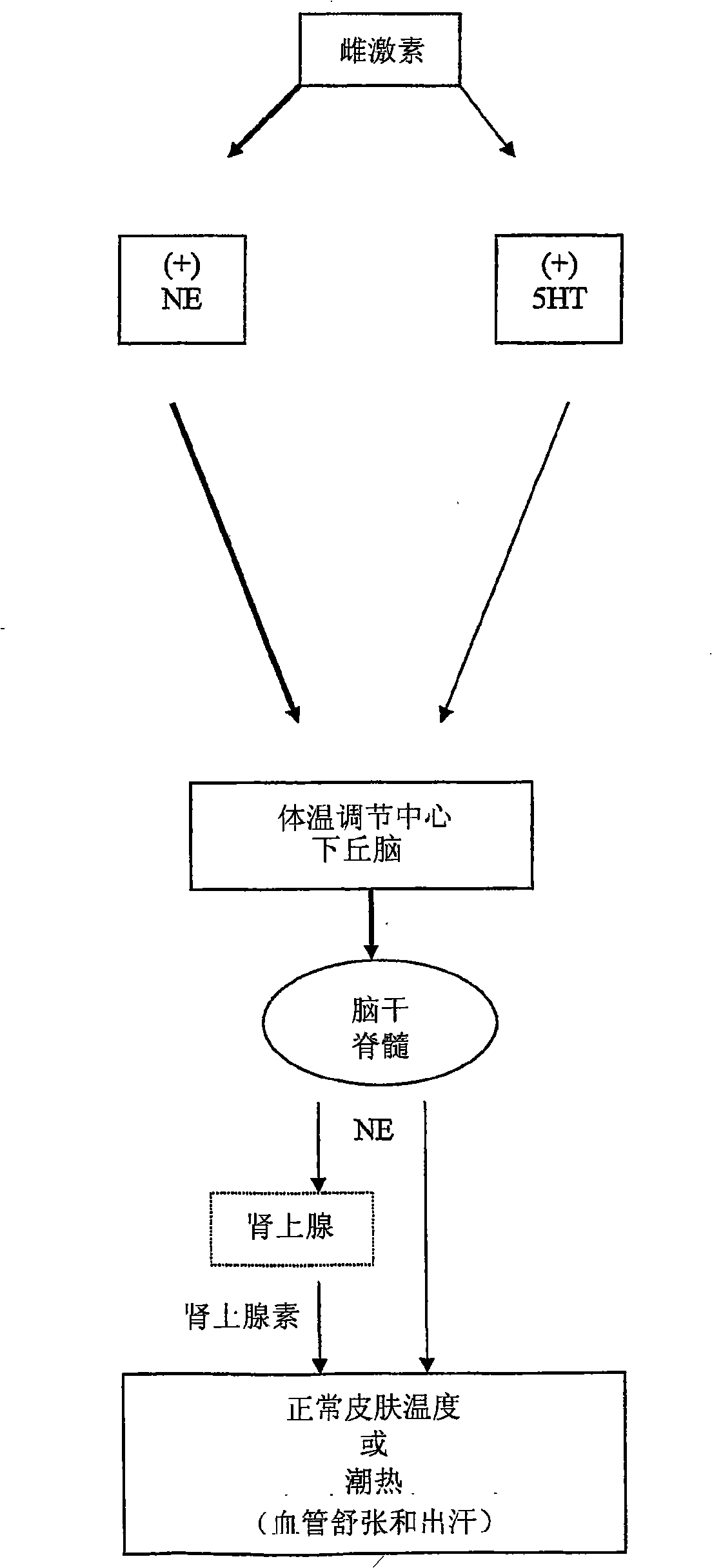
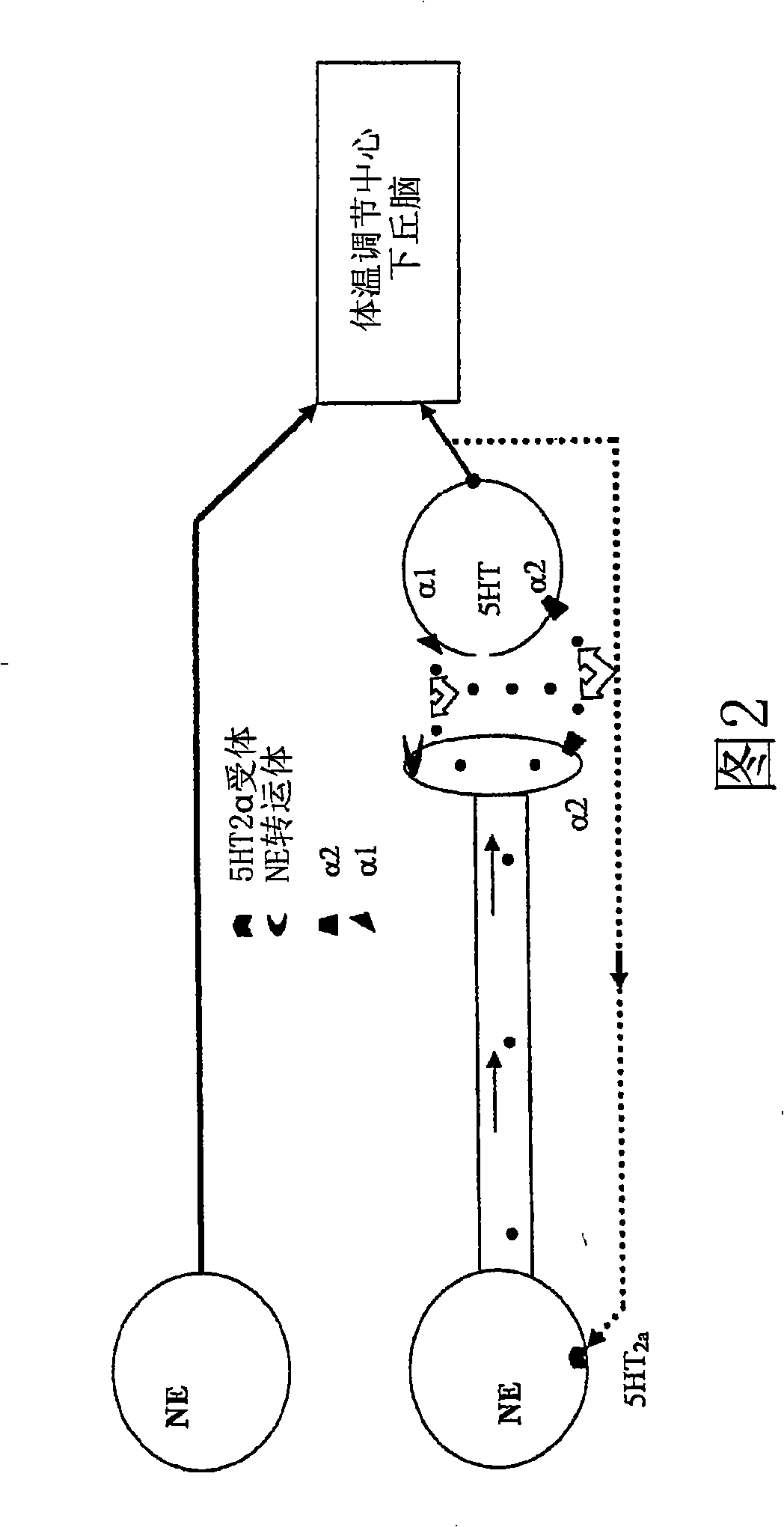
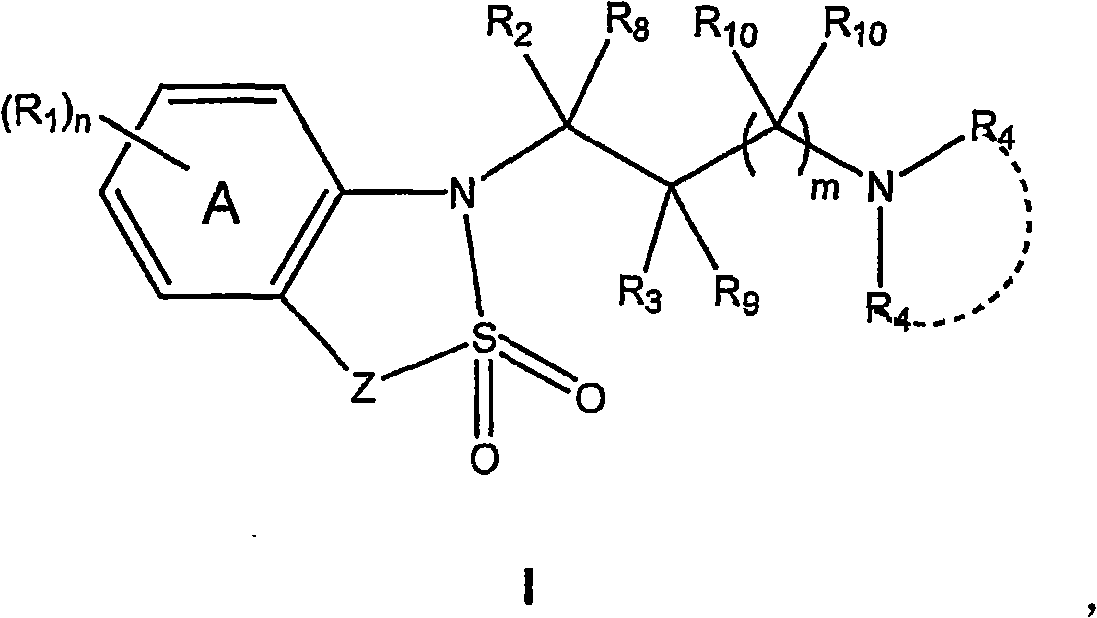
Benzothiadiazolyphenylalkylamine derivatives for use in the treatment of conditions ameliorated by monoamine reuptake
An alkyl, phenyl technology, applied in sexual diseases, drug combinations, nervous system diseases, etc., can solve problems such as unsuitability for women
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0304] Example 1: (3R)-3-[3-(4-Chlorophenyl)-2,2-dioxide-2,1,3-benzothiadiazol-1(3H)-yl]-N-methyl-3 -Phenyl propan-1-amine
[0305]
[0306] 1-(4-chlorophenyl)-1,3-dihydro-2,1,3-benzothiadiazole 2,2-dioxide
[0307]
[0308] Anhydrous diglyme (10 mL) was added to the flask and allowed to reflux vigorously (oil bath, maintained at 190° C.) under a nitrogen atmosphere. N-(4-Chloro-phenyl)-benzene-1,2-diamine (1.09 g, 5.0 mmol) and sulfonamide (0.58 g, 6 mmol) were dissolved in diglyme (5 mL) over 15 minutes ) was added dropwise to the refluxing solution. The mixture was refluxed for another 15 minutes, and then cooled and diluted with ether, washed with 2N HCl, water and saturated brine. The organic layer was separated, dried over anhydrous magnesium sulfate, filtered and concentrated in vacuo. The crude product was dissolved in ether and passed through a plug of silica gel to give 1-(3-bromopropyl)-3-(4-chlorophenyl)-1,3-dihydro-2,1,3-benzothiadiazole 2,2-dioxid...
example 2
[0318] Example 2: (3R)-3-(3-chloro-5-fluorophenyl)-3-(3-isopropyl-2,2-dioxide-2,1,3-benzothiadiazole-1(3H) - base)-N-methylpropan-1-amine hydrochloride
[0319]
[0320] step 1 : A reaction flask (500 mL) containing zinc chloride (6.98 g, 51.2 mmol) was dried by heating under vacuum using a heat gun. After cooling to room temperature, a solution of 3-chloro-5-fluorophenylmagnesium bromide (0.5M in anhydrous THF, 100 mL, 50.0 mmol) was added to the reaction flask via cannula, and the mixture was stirred until all the zinc chloride The solids all dissolved and a viscous bright yellow solution formed (ca. 1 h). A warm water bath (40°C) can be applied to accomplish this. Anhydrous tetrahydrofuran (100 mL) was added followed by tetrakis(triphenylphosphine)palladium (2.89 g, 2.50 mmol, 0.05 equiv). After cooling to 0°C, 3-chloropropionyl chloride (5.05 mL, 52.5 mmol, 1.05 equiv) was added dropwise, and the mixture was stirred at 0°C for 2 hours. The reaction mixture was...
example 3
[0334] Example 3: (3R)-3-(3-chloro-5-fluorophenyl)-N-methyl-3-(3-methyl-2,2-dioxide-2,1,3-benzothiadiazole -1(3H)-yl)propan-1-amine hydrochloride
[0335]
[0336] In a manner similar to Example 2, step 4, from N-methyl-1,2-phenylenediamine prepared as a white solid 1-Methyl -1,3-dihydro-2,1,3-benzothiadiazole 2,2-dioxide . MS(ES) m / z 183.1 ([M-H] - ).
[0337] In a manner similar to Example 2, step 5, from 1-methyl-1,3-dihydro-2,1,3-benzothiadiazole 2,2-dioxide and (1S)-3-chloro- 1-(3-Chloro-5-fluorophenyl)propan-1-ol (Example 2 step 2) was prepared as a viscous colorless liquid 1-[(1R)-3-chloro-1-(3-chloro-5-fluorophenyl)propyl]-3-methyl-1,3-dihydro-2,1,3-benzothiadi Azole 2,2-dioxide .
[0338] In a manner similar to Example 2, Step 6, from 1-[(1R)-3-chloro-1-(3-chloro-5-fluorophenyl)propyl]-3-methyl-1,3-dihydro -2,1,3-Benzothiadiazole 2,2-dioxide prepared as white powder (3R)-3-(3-chloro-5-fluorophenyl)-N-methyl -3-(3-Methyl-2,2-dioxide-2,1,3-benzothia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com