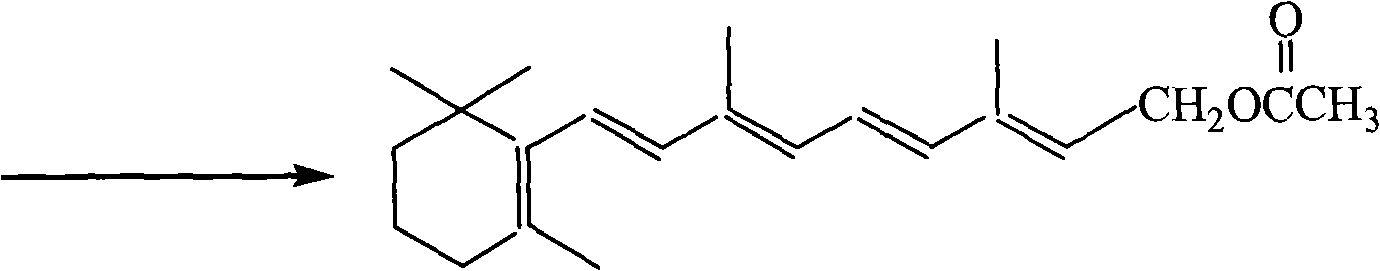
Process for synthesizing 1-chlorine-2-methyl-4-acetoxy-2-butylene
A technology of acetoxy and synthetic methods, applied in the chemical field, can solve problems such as poor product content, poor product yield and content, and increased impurities, and achieve the effects of low cost, mild reaction system, high content and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of 1-chloro-2-hydroxyl-2-methyl-3-butene and 1-chloro-2-methyl-4-hydroxyl-2-butene mixture
[0033] Put a 500ml three-neck bottle equipped with a thermometer and a solid feeding port into an alcohol cooling bath; add 68g (1mol) of isoprene, 100ml of water and 0.5g of inhibitor hydroquinone; Add tetrachloroglycocuril (95% available chlorine) in batches from the feeding port, 56 g (0.2 mol) in total, and add in about 1 hour, then continue to insulate and stir for 1 hour, filter, wash the filter cake with 15 ml of water, and it is a loose white powder solid. The filtrates were combined and allowed to stand for separation. The organic layer was lower than 40°C to recover unreacted isoprene under reduced pressure to obtain 90 g of crude product. Gas phase analysis showed that the total content of the product was 92.5%, and the yield was 92.1%. It can be directly used in the next reaction; the water layer is 99.5 grams, and it will be used in the next rea...
Embodiment 2
[0034] Embodiment 2: material proportioning, operating temperature and aftertreatment are with embodiment 1, and difference is that reaction water is the waste water layer in embodiment 1, gets crude product 93g, gas phase analysis shows that product total content is 89.5%, yield 92.1%. The filter cake is a loose white powder solid, and the water layer is 98 grams.
Embodiment 3
[0035] Embodiment 3: material proportioning, operating temperature and aftertreatment are the same as embodiment 1, the difference is that the water for reaction is the waste water layer in embodiment 2, obtains crude product 94.5g, gas phase analysis shows that product total content is 90.5%, yield 94.6% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com