Method for synthesizing anisyl methyl ketone
A methoxyacetophenone and methoxylation technology, applied in the chemical industry, can solve the problems of high regeneration temperature of HZSM-5 molecular sieve, high experimental cost, low recovery rate, etc., and achieve good promotion value and high experimental yield. , the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Put 47.05g of phenol, 100g of acetonitrile and 80g of 35% hydrochloric acid into a 250ml four-neck flask, heat to 100°C, and observe the changes of reactants and products. After reacting for 2h to the end point, neutralize, cool and crystallize, filter and dry.
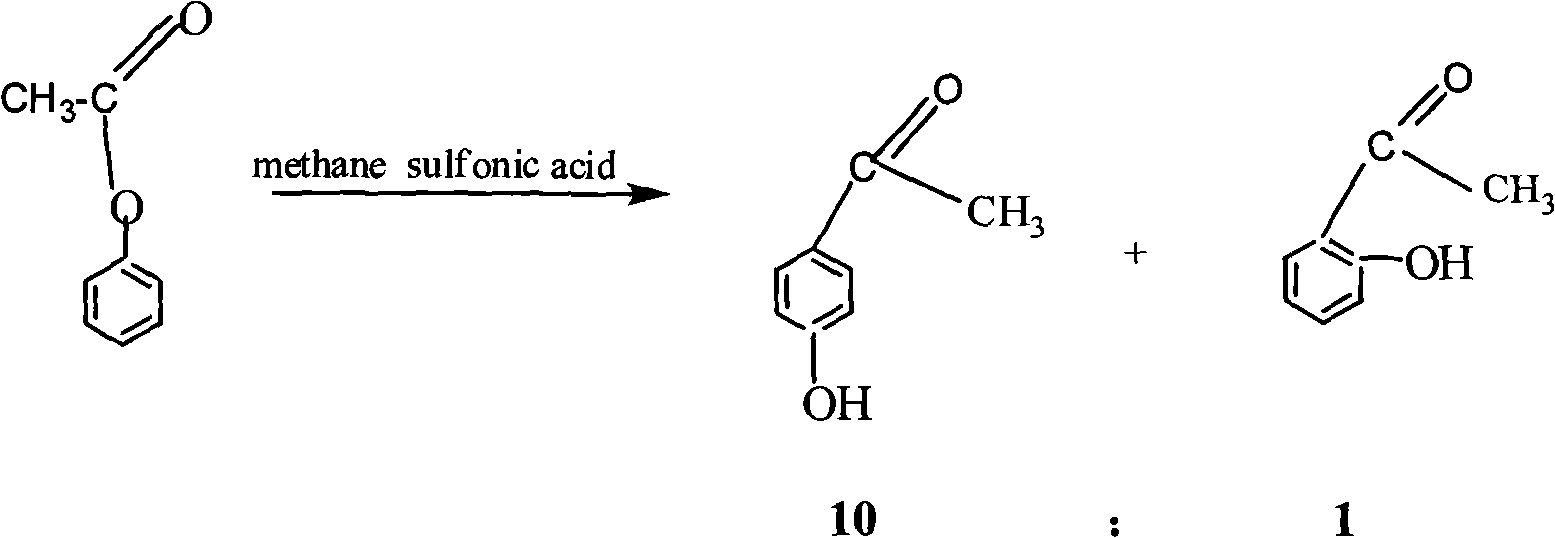
[0041] Put 50g of the above-mentioned crystals, 150g of toluene and 20g of methanesulfonic acid into a 250ml four-neck flask, heat to 90°C, and react for 2.5h. Cool to 20°C, crystallize, filter, and dry to obtain coarse crystals, and the filtrate is recycled.
[0042] The crude crystals were recrystallized at a recrystallization temperature of 108° C., filtered, and dried to obtain p-acetylphenol with a purity of 98.6%.
[0043] Take 30g of p-acetylphenol, dissolve it in aqueous sodium hydroxide solution, adjust the pH to about 10, and the temperature is 45°C, slowly add 32g of dimethyl sulfate, and react for 2.5h to obtain 28.8g of colorless crystals with strong fragrance, melting point 37.4-38.6°C.
[0044...
Embodiment 2
[0046] Put 45g of phenol, 95g of acetonitrile and 75g of 40% sulfuric acid into a 250ml four-neck flask, heat to 96°C, and observe the changes of reactants and products. Reaction 1.5h. Neutralize, cool and crystallize, filter and dry.
[0047] Put 46g of the above-mentioned crystals, 150g of toluene and 20g of methanesulfonic acid into a 250ml four-neck flask, heat to 88°C, and react for 2h. Cool to 20°C, crystallize, filter, and dry to obtain coarse crystals, and the filtrate is recycled.
[0048] The crude crystals were recrystallized at a recrystallization temperature of 109° C., filtered, and dried to obtain p-acetylphenol with a purity of 99.2%.
[0049]Take 28g of p-acetylphenol, dissolve it in aqueous sodium hydroxide solution, adjust the pH to about 11, and the temperature at 42°C, and slowly add 31g of dimethyl sulfate. After reacting for 3 hours, 27.6 g of colorless crystals with a strong aroma were obtained, with a melting point of 37.7-38.6°C.
[0050] The melt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com