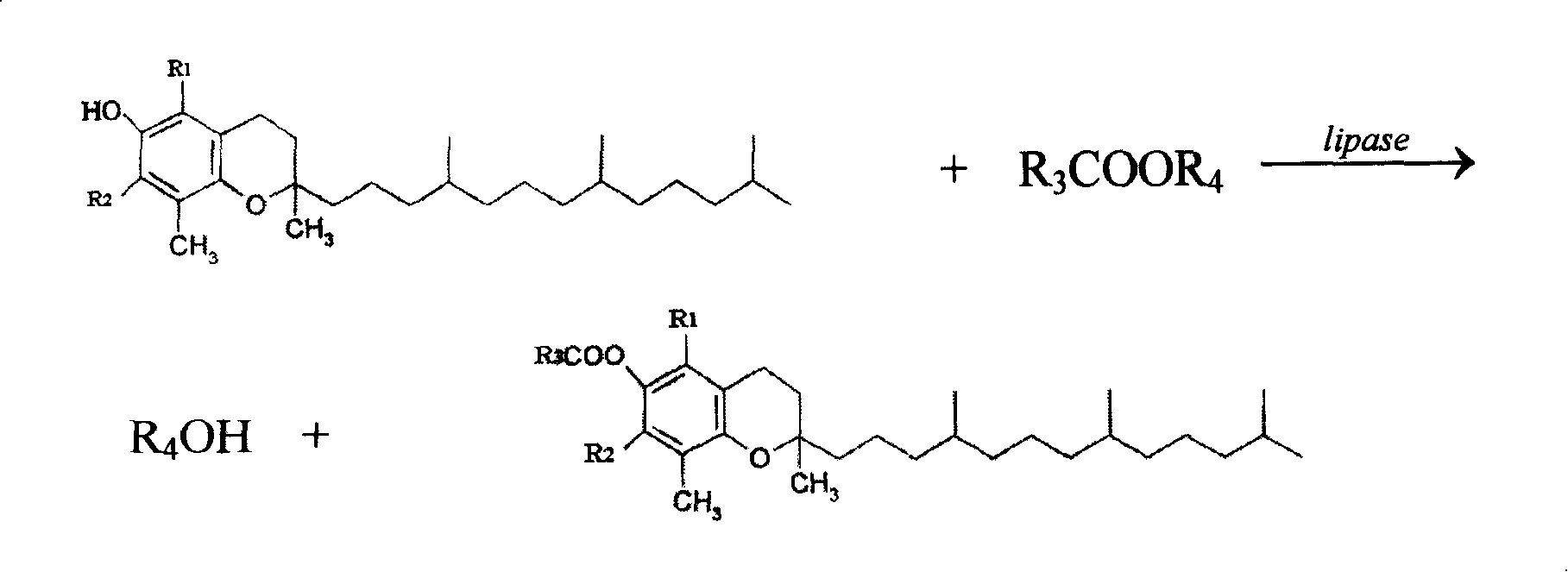
Method for synthesizing natural tocopherol ester catalyzed by lipase
A lipase and tocopherol technology, which is applied in the field of lipase-catalyzed synthesis of natural tocopherol esters, can solve the problems of many toxic by-products, large waste water, and difficulty in continuous production, and achieve the effects of low toxicity, long life and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]Two kinds of substrates 0.43g (1mmol) natural α-tocopherol and 0.185ml (2mmol) ethylene acetate are joined in the sherwood oil of 0.5% water content in reaction medium 10ml, react with 0.02g free Candida lipase (10000U / g) as a catalyst. Under the condition of temperature of 40° C., the shaking table was oscillated (180 r / min), and the reaction conversion rate reached 50.00% after 72 hours of reaction. After the reaction was completed, stand at room temperature and filter to separate free lipase. The organic solvent is distilled off from the reaction solution under a vacuum degree of 0.05-0.08 MPa to obtain the product natural tocopheryl ester.
Embodiment 2
[0037] The operation steps are the same as in Example 1, except that the catalyst is changed from free Candida lipase to Penicillium lipase, and the conversion rate is 45.21%.
Embodiment 3
[0039] Two kinds of substrates 0.43g (1mmol) natural α-tocopherol and 0.185ml (2mmol) ethylene acetate are added in reaction medium 10ml water content and be 0.5% petroleum ether, the lipase immobilized with 0.2g textile cotton cloth is reacted (250U / g) is the catalyst. Under the condition of temperature 40° C., the shaking table was oscillated (180 r / min), and the reaction conversion rate reached 91.03% after 72 hours of reaction. The immobilized lipase is taken out, the reaction solution is filtered, and the organic solvent is evaporated under a vacuum of 0.05-0.08 MPa to obtain the product natural tocopheryl ester.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com