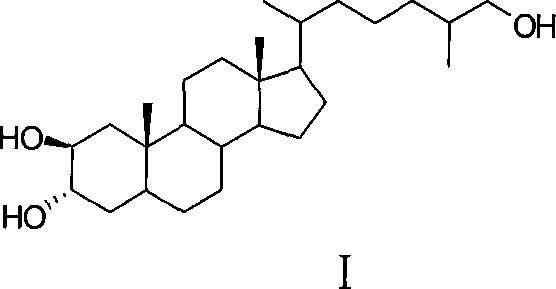
Synthesis of polyhydroxy ocean steroid (25R)-5 alpha-cholesteric-2 beta,3 alpha,26-triol
A synthetic method and polyhydric technology, applied in the direction of steroids, organic chemistry, etc., can solve the problem of low content and achieve a scientific and reasonable synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
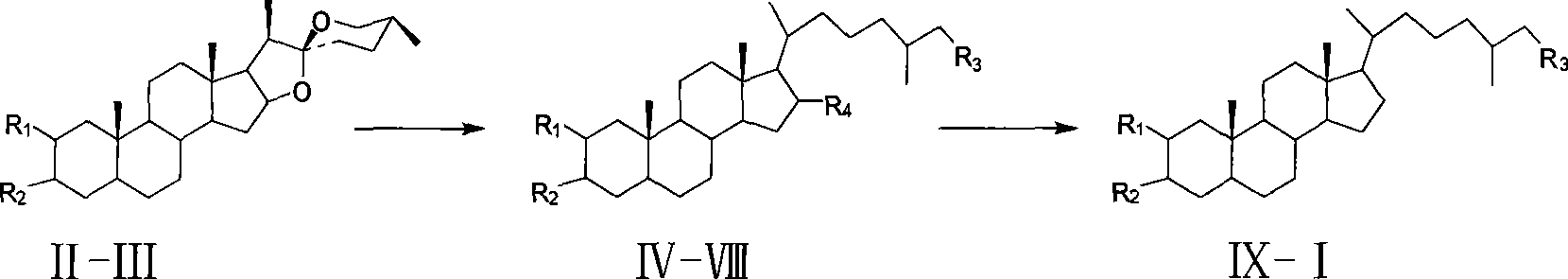
[0022]The method for synthesizing (25R)-5α-cholester-2β,3α,26-triol provided in Example 1 includes the following steps:
[0023] (1). Dissolve 50g (0.12mol) of Tignogenin II in 250ml of dry pyridine, add 45.8g (0.24mol) of p-toluenesulfonyl chloride, and stir for 24h at room temperature. The reaction solution was slowly poured into 1000 ml of 17% (w / w) HCl, a white precipitate was separated out, filtered with suction, the precipitate was washed with water to neutrality, and dried to obtain 68 g of white solid compound III with a yield of 99%.
[0024] (2). 30g (0.053mol) of compound III is dissolved in 450ml of dry DMF, and 45.6g (0.53mol) of LiBr and Li are added. 2 CO 3 39.2g (0.53mol), reflux for 1.5h. After cooling to room temperature, it was slowly poured into 10% (w / w) HCl, filtered with suction, the precipitate was washed with water to neutrality, and dried to obtain 23g of white solid powder compound IV. The yield was 87.8%.
[0025] (3). In a 2L three-necked round bottom ...
Embodiment 2
[0032] The synthesis method of (25R)-5α-cholester-2β,3α,26-triol provided in Example 2 includes the following steps:
[0033] (1). Same as Example 1.(1).
[0034] (2). 30g (0.053mol) of compound III is dissolved in 450ml of dry DMF, and 45.6g (0.53mol) of LiBr and Li are added. 2 CO 3 39.2g (0.53mol), refluxed at 110°C for 4h. After cooling to room temperature, it was slowly poured into 10% (w / w) HCl, filtered with suction, the precipitate was washed with water to neutrality, and dried to obtain 23g of white solid powder compound IV, with a yield of 82%.
[0035] (3)~(8). Same as Example 1(3)~(8).
Embodiment 3
[0037] The synthesis method of (25R)-5α-cholester-2β,3α,26-triol provided in Example 3 includes the following steps:
[0038] (1)~(2). Same as Example 1.(1)~(2).
[0039] (3). In a 2L three-necked round bottom flask equipped with mechanical stirring, constant pressure dropping funnel and reflux condenser, add compound IV (15g, 37.7mmol) and 1100ml ethanol in turn, add 445g zinc amalgam, and drop under reflux 300ml of concentrated hydrochloric acid was added, and the reaction was followed by TLC, and the reaction was stopped when the raw materials disappeared. Filter, remove most of the ethanol under reduced pressure, extract 250 ml×3 times with dichloromethane, wash the organic layer with water until it is neutral, dry with anhydrous sodium sulfate, and concentrate to obtain 13 g of white solid. Purified by silica gel column chromatography (ethyl acetate: petroleum ether, V:V=3:7), 10.8 g of white solid compound V was obtained, with a yield of 65%.
[0040] (4)~(8). Same as Exampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com