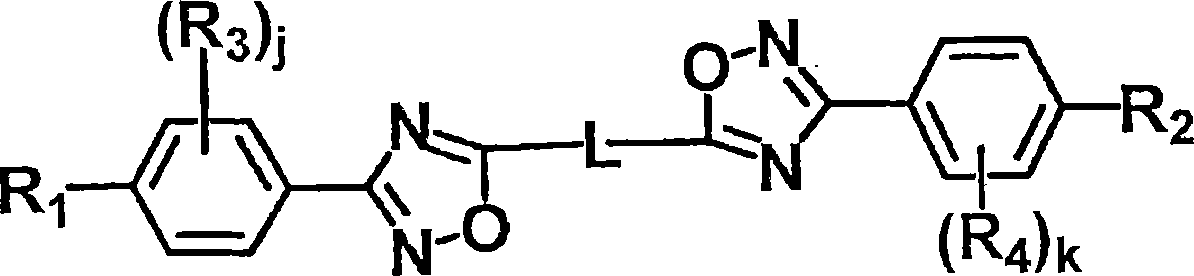
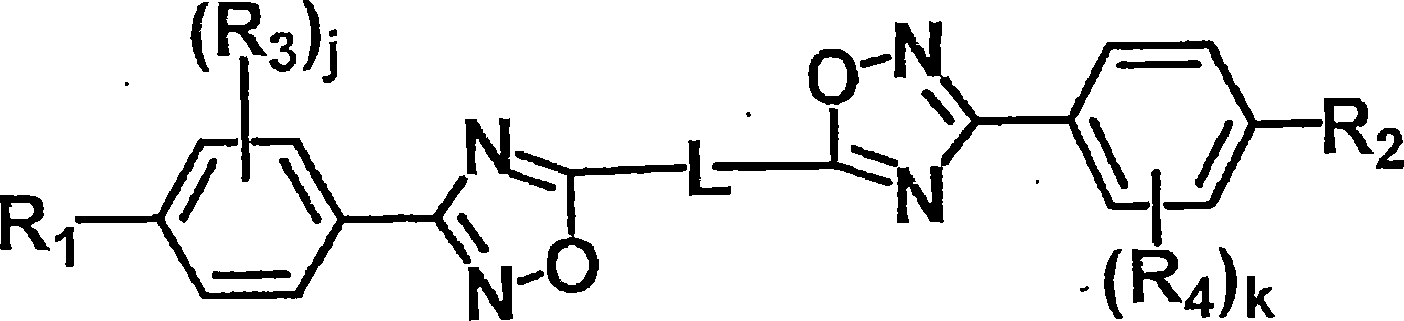
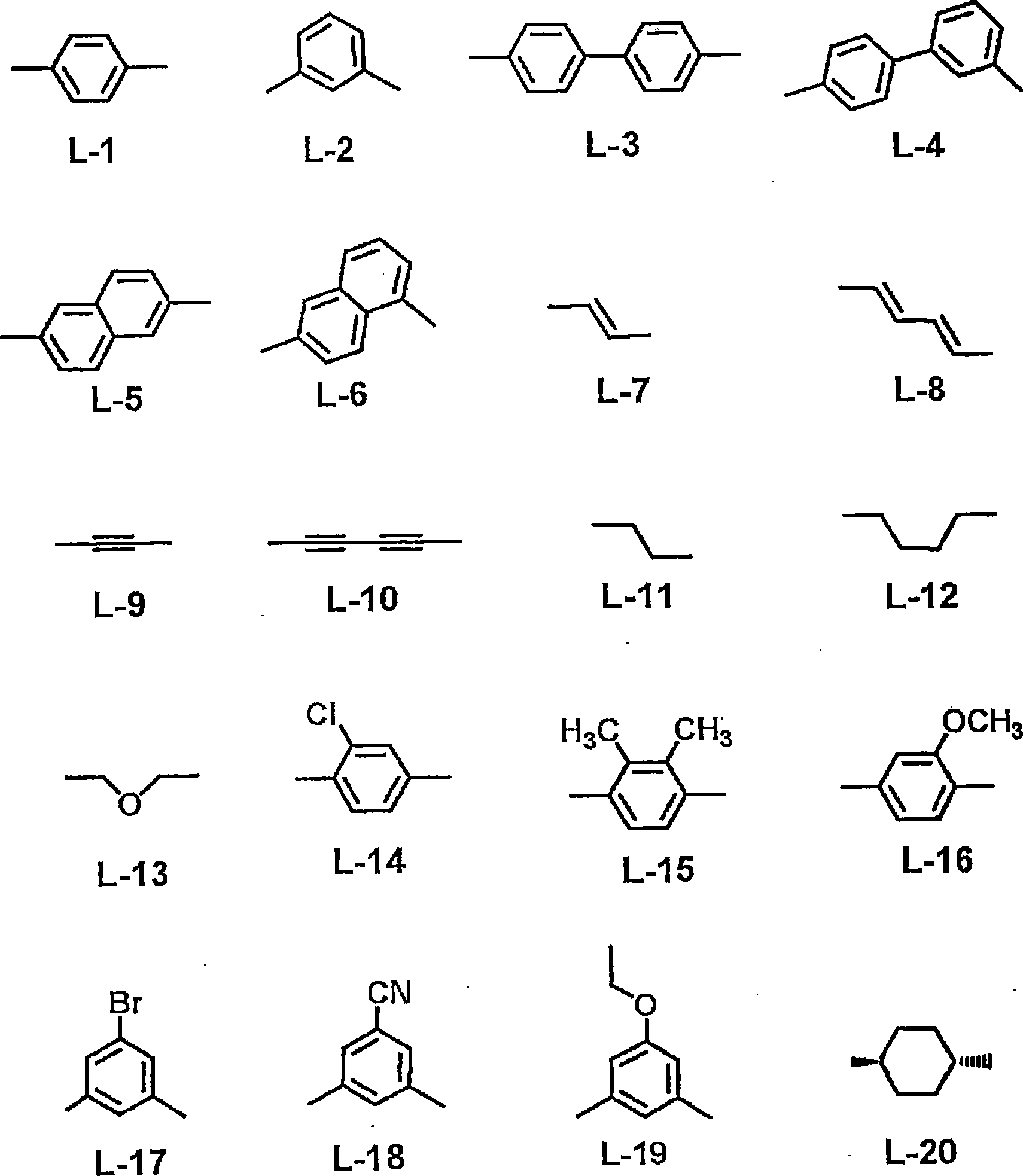
Liquid crystal compound, liquid crystal composition, thin film, and liquid crystal display
A liquid crystal compound and liquid crystal phase technology, applied in liquid crystal materials, chemical instruments and methods, instruments, etc., can solve the problems of orientation disorder and enlargement, and achieve the effect of wide viewing angle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0129] (Synthesis of Example Compound (1))
[0130] Example compound (1) was synthesized according to the following scheme.
[0131]
[0132] At room temperature, 139 g (2.1 mol) of 50% hydroxylamine aqueous solution was added dropwise into 144.0 g (1.05 mol) of 2-fluoro-4-cyanophenol in methanol solution. The temperature of the reaction system was gradually raised and stirred under reflux for 3 hours. After the reaction, it was ice-cooled, and cold water was added to the reaction system for crystal precipitation. The crystals thus obtained were collected by filtration and dried to obtain 139.5 g of (1-A) (yield 82%).
[0133] At room temperature, 8.1ml (0.1mol) of pyridine was added to 300ml of N,N-dimethylacetamide containing 17g (0.1mol) (1-A), and 6.7g (33mmol) of Phthaloyl chloride. After the addition, it was stirred at room temperature for 30 minutes, then the reaction temperature was raised to 100°C. Stir like this for 3 hours, then cool naturally. When methano...
Embodiment 2
[0138] (Synthesis of Example Compound (2))
[0139] Example compound (2) was synthesized according to the following scheme.
[0140]
[0141] Example compound (2) was obtained through the same process, except that the octyl chloroformate in Example 1 was changed to 2-methylpropyl chloroformate.
[0142] The phase transition temperature of Example Compound (2) thus obtained was determined by DSC measurement, and the crystal structure was observed under a polarizing microscope, whereby the following results were obtained.
[0143]
Embodiment 3
[0145] (Synthesis of Example Compound (3))
[0146] Example compound (3) was synthesized according to the following scheme.
[0147]
[0148] Example compound (3) was obtained through the same process, except that the octyl chloroformate in Example 1 was changed to 4-acryloylbutyl chloroformate.
[0149] The phase transition temperature of Example Compound (3) thus obtained was determined by DSC measurement, and the crystal structure was observed under a polarizing microscope, whereby the following results were obtained.
[0150]
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com