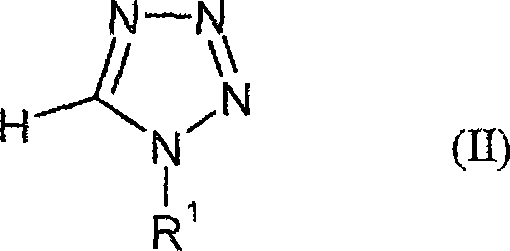
1-cycloalkyl-5-iodotetrazoles
A compound and cycloalkyl technology, applied in organic chemistry, drug combination, animal repellent, etc., can solve problems that do not mention biological effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0205] 1-cyclopentyl-5-iodotetrazole
[0206] In an inert gas environment, 10.42 mmol of butyllithium (1.6 M hexane solution ), so that the temperature of the reaction mixture does not exceed -70°C. The mixture was stirred at this temperature for 30 minutes, then 4 ml of a solution of 8.68 mmol (2.2 g) of iodine in anhydrous tetrahydrofuran were added dropwise. After stirring at this temperature for 30 minutes, the reaction mixture was heated to 23°C and water was added carefully. The solvent was extracted with ethyl acetate and the combined organic phases were washed with sodium thiosulfate solution and saturated sodium chloride solution and dried over sodium sulfate. The solid obtained by distilling off the solvent under reduced pressure was stirred with diisopropyl ether to obtain 1.55 g (68%) of 1-cyclopentanol-5-iodotetrazole having a melting point of 119°C.
[0207] The preparation method of the compound listed in table 1 is similar to example 1:
[0208]
[0209]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com