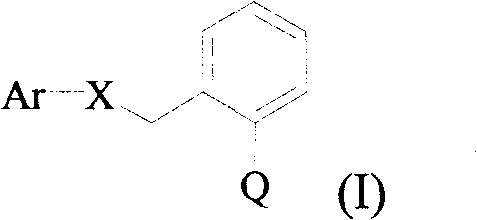
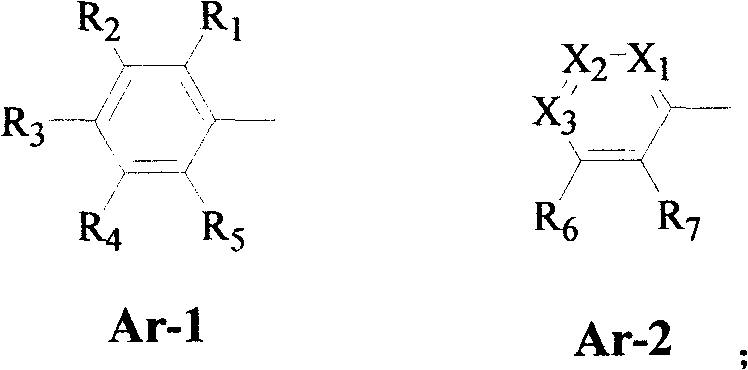
Substituted aryl ethers compounds as well as preparation and uses thereof
A technology of compounds and aryl ethers, applied in the fields of agricultural insecticides and fungicides, can solve the problems that substituted aryl ether compounds are not specifically disclosed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0151] Example 1: Preparation of compound 40
[0152]
[0153] At room temperature, the mixture containing 0.8 g of anhydrous potassium carbonate, 0.39 g of compound (II-1), and 0.4 g of compound (III-1) in 20 ml of N,N-dimethylformamide was heated under reflux and stirred for reaction 5 After hours, the reaction mixture was poured into ice water, extracted with ethyl acetate 3 times, the extracts were combined, washed with saturated brine 3 times, dried, filtered, and concentrated under reduced pressure to obtain a light yellow solid as a crude product. Column chromatography with a mixture of ethyl acetate and petroleum ether (1:8) yielded 0.51 g of the title compound, melting point 84-86°C. The yield was 70.5%.
[0154] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0155] δppm 3.71 (3H, s), 3.84 (3H, s), 5.10 (2H, s), 7.01 (1H, m), 7.20 (1H, m), 7.25 (1H, m), 7.35 (2H, m), 7.56 (1H, m), 7.62 (1H, s).
example 2
[0156] Example 2: Preparation of compound 193
[0157]
[0158] At room temperature, the mixture containing 0.8 g of anhydrous potassium carbonate, 0.39 g of compound (II-2), 0.42 g of compound (III-2) in 20 ml of N,N-dimethylformamide was heated under reflux and stirred for 5 hours. , Pour the reaction mixture into ice water, extract 3 times with ethyl acetate, combine the extracts, wash 3 times with saturated brine, dry, filter, and concentrate under reduced pressure to obtain a pale yellow solid as a crude product. Column chromatography with a mixture of ethyl acetate and petroleum ether (1:8) gave the title compound 0.53 g, melting point 92-94°C. The yield was 73.2%.
[0159] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0160] δppm 3.86 (3H, s), 4.04 (3H, s), 5.06 (2H, s), 7.06 (1H, d), 7.15 (1H, m), 7.23 (1H, m), 7.44 (3H, m), 7.58 (1H, m).
example 3
[0161] Example 3: Preparation of Compound 323
[0162]
[0163] 0.60 g of compound 193 was stirred in 50 ml of tetrahydrofuran at room temperature with twice the molar ratio of methylamine aqueous solution overnight, concentrated and then extracted with ethyl acetate twice, the combined extracts were washed with water three times, and then with saturated brine twice. Dry, filter, and concentrate to obtain 0.53 g of the title compound, melting point 132-134°C. The yield was 88.5%.
[0164] NMR data ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0165] δppm 2.92 (3H, s), 3.92 (3H, s), 5.12 (2H, s), 6.95 (1H, d), 7.24 (1H, m), 7.42 (3H, m), 7.54 (1H, d), 7.62(1H, s).
[0166] Other compounds of the present invention can be synthesized by referring to the above methods.
[0167] Part of the nuclear magnetic data of the compounds of general formula I shown in Table 1 and Table 2 ( 1 HNMR, 300MHz, internal standard TMS, solvent CDCl 3 )as follows:
[0168] Compound 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com