Method for crystal system conversion of zopiclone
A technology of zopiclone and conversion method, which is applied in the field of crystal form conversion of compounds, can solve the problems of low yield, difficulty in ensuring safety, difficulty in producing zopiclone, etc., and achieve high yield, safe and reliable production, large The effect of implementing value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Put 20g of zopiclone of crystal form C and 80g of isopropanol into a 500ml three-necked flask, raise the temperature to 55°C, keep it for 1.0 hour, then lower the temperature to 0°C, filter, and dry to obtain 18.5g of crystal form A Zopiclone, the yield is 92.5%, the melting point is about 177°C (decomposes at the same time when melting).
[0051] The obtained crystal form A Zopiclone has the following properties:
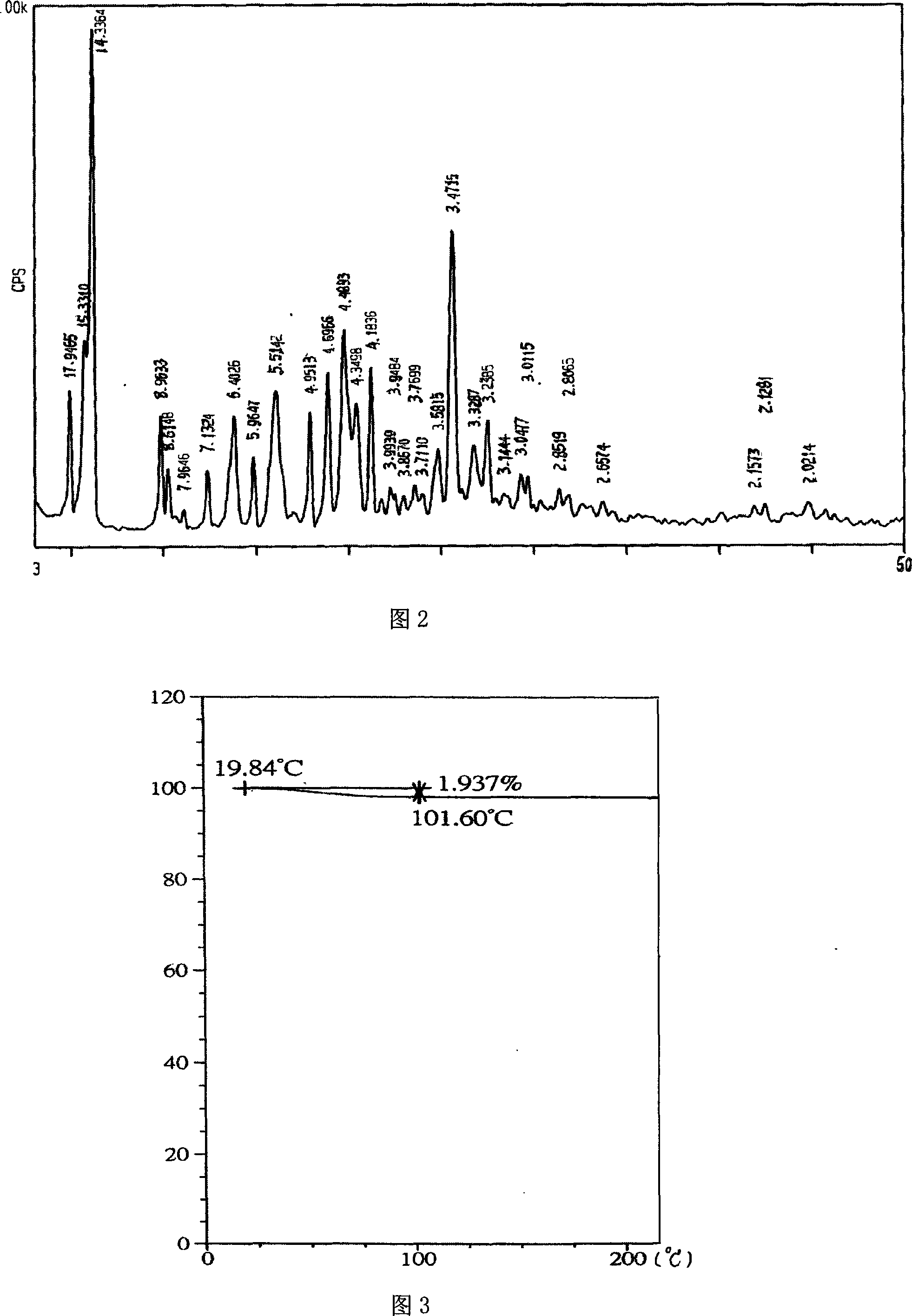
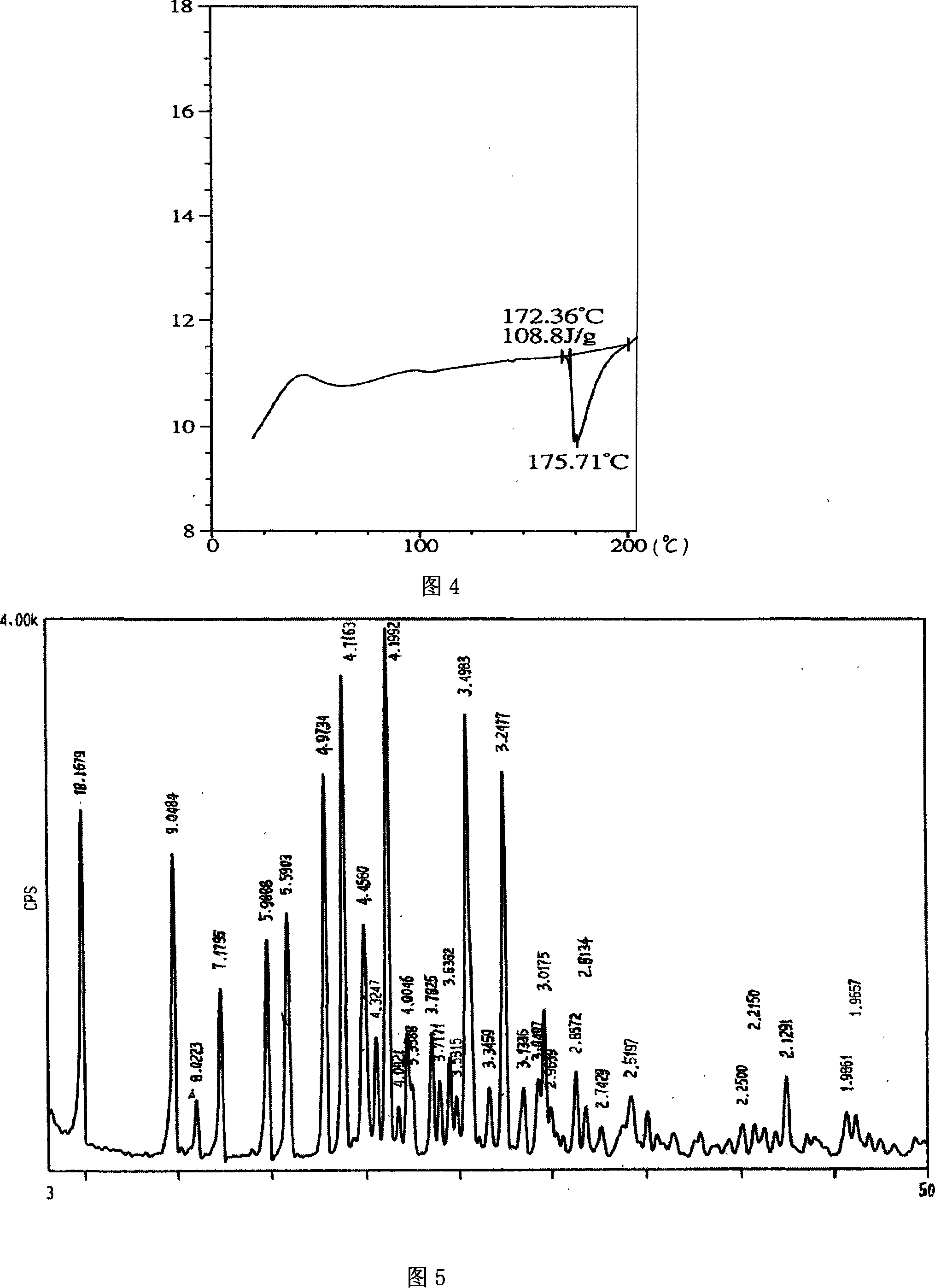
[0052] (1) X-ray powder diffraction (XRPD) (see Figure 5) curve is consistent with the curve of crystal form A in Figure 1.
[0053] (2) The thermogravimetric analysis (TGA) curve (see Figure 6) is a straight line with no weight change (before 130°C).
[0054] (3) The differential scanning calorimetry (DSC) curve (see Figure 7) has only one endothermic peak, and its maximum endothermic peak is about 177°C.
Embodiment 2
[0056] 20g of zopiclone of crystal form C and 120g of isopropanol were put into a 500ml three-necked flask, the temperature was raised to reflux, after holding for 0.5 hours, the temperature was lowered to 0°C, filtered, and dried to obtain 18.2g of zopiclone of crystal form A. Picclonal, the yield is 91.0%, the melting point is about 177°C (decomposes while melting). The properties of the crystal form C zopiclone raw material and the obtained crystal form A zopiclone are the same as in Example 1.
Embodiment 3
[0058] 20g of zopiclone of crystal form C and 160g of isopropanol were put into a 500ml three-necked flask, heated to reflux, after holding for 0.5 hours, cooled to 0°C, filtered and dried to obtain 18.4g of zopiclone of crystal form A Picclonal, the yield is 92.0%, the melting point is about 177°C (decomposes at the same time when melting). The properties of the crystal form C zopiclone raw material and the obtained crystal form A zopiclone are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com